Evaluating the oxysterol combination of 22(S)-hydroxycholesterol and 20(S)-hydroxycholesterol in periodontal regeneration using periodontal ligament stem cells and alveolar bone healing models
- PMID: 29208033
- PMCID: PMC5717822
- DOI: 10.1186/s13287-017-0725-9
Evaluating the oxysterol combination of 22(S)-hydroxycholesterol and 20(S)-hydroxycholesterol in periodontal regeneration using periodontal ligament stem cells and alveolar bone healing models
Abstract
Background: Oxysterols, oxygenated by-products of cholesterol biosynthesis, play roles in various physiological and pathological systems. However, the effects of oxysterols on periodontal regeneration are unknown. This study investigated the effects of the specific oxysterol combination of 22(S)-hydroxycholesterol and 20(S)-hydroxycholesterol (SS) on the regeneration of periodontal tissues using in-vitro periodontal ligament stem cells (PDLSCs) and in-vivo models of alveolar bone defect.
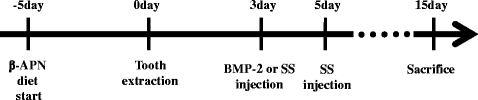
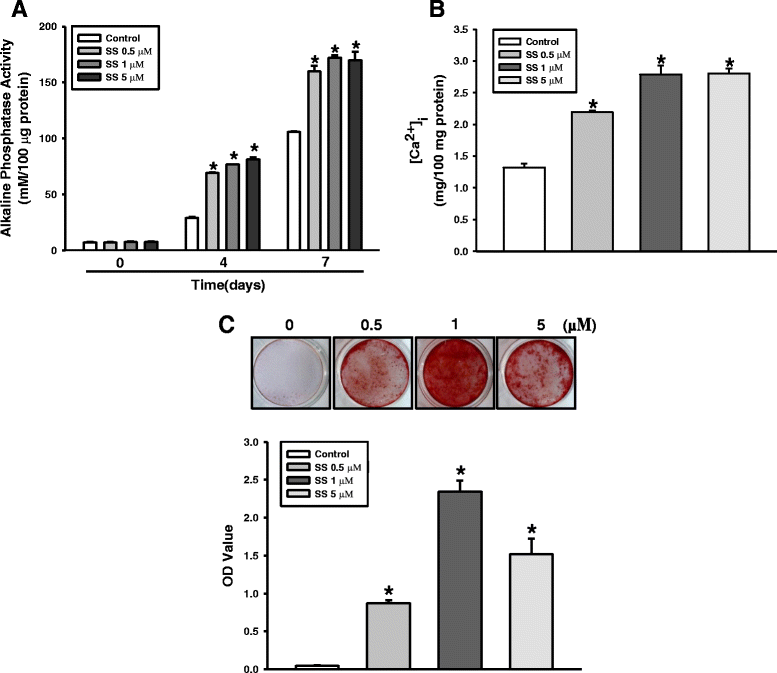
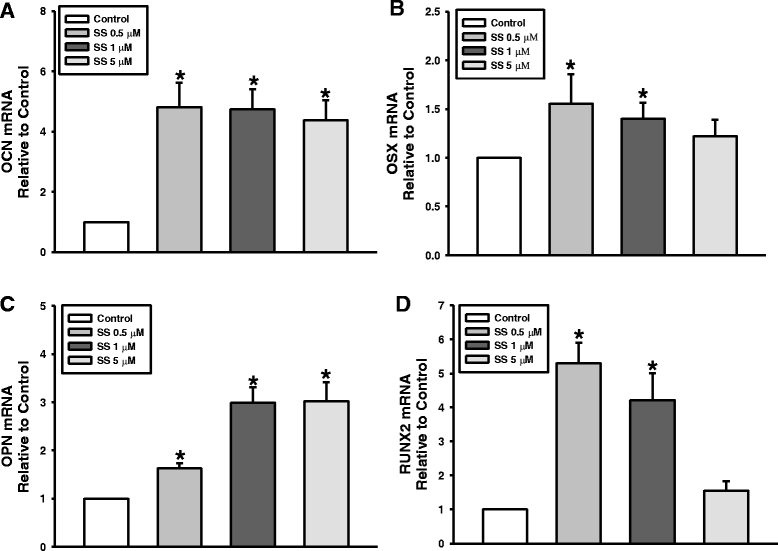
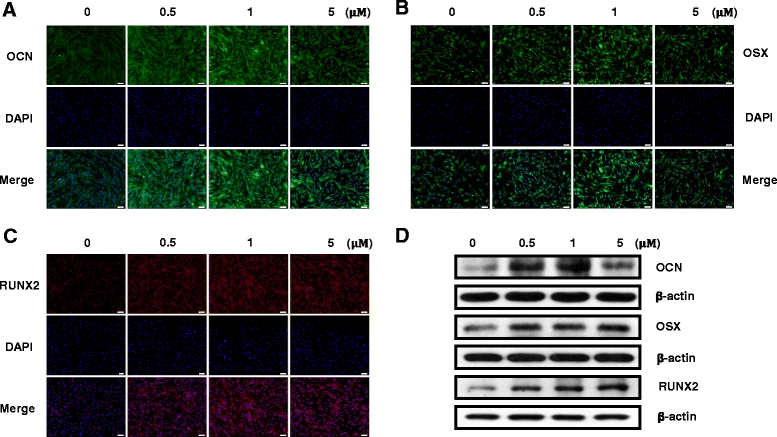
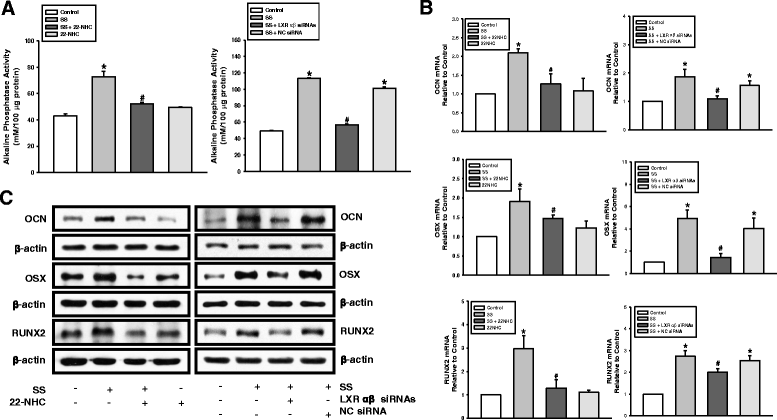
Methods: To evaluate the effects of the combined oxysterols on PDLSC biology, we studied the SS-induced osteogenic differentiation of PDLSCs by assessing alkaline phosphatase activity, intracellular calcium levels [Ca2+]i, matrix mineralization, and osteogenic marker mRNA expression and protein levels. To verify the effect of oxysterols on alveolar bone regeneration, we employed tooth extraction bone defect models.
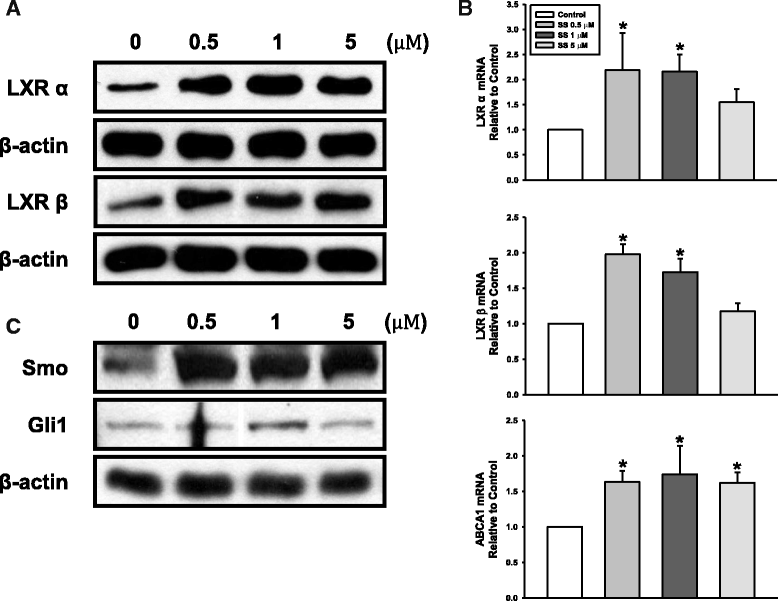
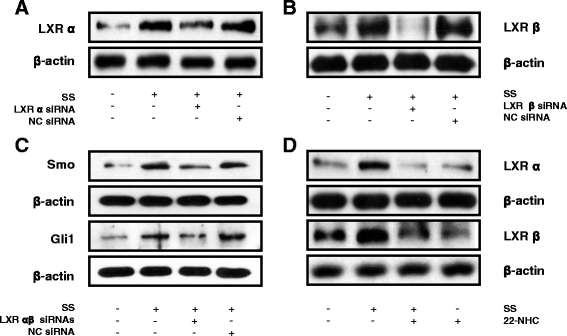
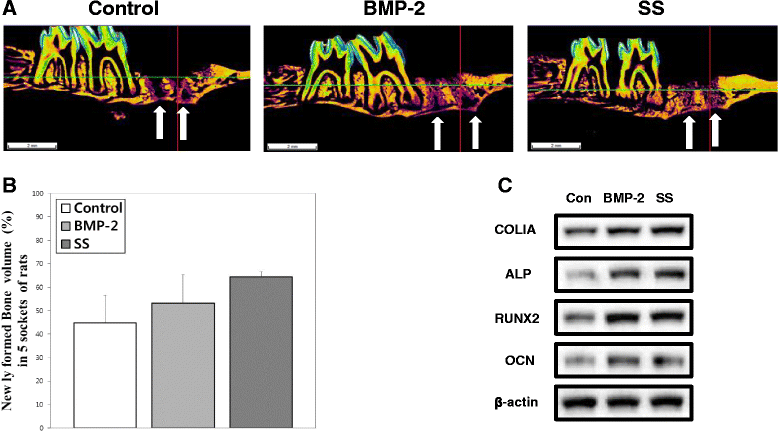
Results: Oxysterols increased the osteogenic activity of PDLSCs compared with the control group. The expression of liver X receptor (LXR) α and β, the nuclear receptors for oxysterols, and their target gene, ATP-binding cassette transporter A1 (ABCA1), increased significantly during osteogenesis. Oxysterols also increased protein levels of the hedgehog (Hh) receptor Smo and the transcription factor Gli1. We further confirmed the reciprocal reaction between the LXRs and Hh signaling. Transfection of both LXRα and LXRβ siRNAs decreased Smo and Gli1 protein levels. In contrast, the inhibition of Hh signaling attenuated the LXRα and LXRβ protein levels. Subsequently, SS-induced osteogenic activity of PDLSCs was suppressed by the inhibition of LXRs or Hh signaling. The application of SS also enhanced bone formation in the defect sites of in-vivo models, showing equivalent efficacy to recombinant human bone morphogenetic protein-2.
Conclusions: These findings suggest that a specific combination of oxysterols promoted periodontal regeneration by regulating PDLSC activity and alveolar bone regeneration.
Keywords: Alveolar bone defect; Bone regeneration; Osteogenic differentiation; Oxysterol; Periodontal ligament stem cells.
Conflict of interest statement
Ethics approval and consent to participate
All subjects involved in this study were informed about its purpose and procedures, and the study was approved by the Kyung Hee University Review Board. Written informed consent was obtained from all donors or their guardians on behalf of minor participants. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Kyung Hee University Hospital at Gangdong (KHNMC AP 2016-002).
Consent for publication
All authors consented to publication of the present manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Mitochondrial function contributes to oxysterol-induced osteogenic differentiation in mouse embryonic stem cells.Biochim Biophys Acta. 2015 Mar;1853(3):561-72. doi: 10.1016/j.bbamcr.2014.12.011. Epub 2014 Dec 16. Biochim Biophys Acta. 2015. PMID: 25523141
-
Circular RNA CDR1as regulates osteoblastic differentiation of periodontal ligament stem cells via the miR-7/GDF5/SMAD and p38 MAPK signaling pathway.Stem Cell Res Ther. 2018 Aug 31;9(1):232. doi: 10.1186/s13287-018-0976-0. Stem Cell Res Ther. 2018. PMID: 30170617 Free PMC article.
-
Oxysterols enhance osteoblast differentiation in vitro and bone healing in vivo.J Orthop Res. 2007 Nov;25(11):1488-97. doi: 10.1002/jor.20437. J Orthop Res. 2007. PMID: 17568450
-
Comparative Dental Pulp Stem Cells (DPSCs) and Periodontal Ligament Stem Cells (PDLSCs): Difference in effect of aspirin on osteoblast potential of PDLSCs and DPSCs.Tissue Cell. 2025 Jun;94:102776. doi: 10.1016/j.tice.2025.102776. Epub 2025 Feb 21. Tissue Cell. 2025. PMID: 40022908 Review.
-
Epigenetic regulation of osteogenic differentiation of periodontal ligament stem cells in periodontitis.Oral Dis. 2023 Oct;29(7):2529-2537. doi: 10.1111/odi.14491. Epub 2023 Jan 13. Oral Dis. 2023. PMID: 36582112 Review.
Cited by
-
Periodontal ligament stem cell-based bioactive constructs for bone tissue engineering.Front Bioeng Biotechnol. 2022 Dec 2;10:1071472. doi: 10.3389/fbioe.2022.1071472. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36532583 Free PMC article. Review.
-
Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems.ACS Biomater Sci Eng. 2023 Sep 11;9(9):5222-5254. doi: 10.1021/acsbiomaterials.3c00609. Epub 2023 Aug 16. ACS Biomater Sci Eng. 2023. PMID: 37585562 Free PMC article. Review.
-
Effects of 20(S)-hydroxycholesterol on satellite cell proliferation and differentiation of broilers.Poult Sci. 2021 Feb;100(2):474-481. doi: 10.1016/j.psj.2020.10.032. Epub 2020 Nov 4. Poult Sci. 2021. PMID: 33518099 Free PMC article.
-
7-Ketocholesterol Effects on Osteogenic Differentiation of Adipose Tissue-Derived Mesenchymal Stem Cells.Int J Mol Sci. 2024 Oct 23;25(21):11380. doi: 10.3390/ijms252111380. Int J Mol Sci. 2024. PMID: 39518932 Free PMC article.
-
Bioinformatics Analysis Identified miR-584-5p and Key miRNA-mRNA Networks Involved in the Osteogenic Differentiation of Human Periodontal Ligament Stem Cells.Front Genet. 2021 Sep 27;12:750827. doi: 10.3389/fgene.2021.750827. eCollection 2021. Front Genet. 2021. PMID: 34646313 Free PMC article.
References
-
- Sakaguchi K, Katagiri W, Osugi M, Kawai T, Sugimura-Wakayama Y, Hibi H. Periodontal tissue regeneration using the cytokine cocktail mimicking secretomes in the conditioned media from human mesenchymal stem cells. Biochem Biophys Res Commun. 2017;484(1):100–6. doi: 10.1016/j.bbrc.2017.01.065. - DOI - PubMed
-
- Kl V, Ryana H, Dalvi PJ. Autologous periodontal stem cell assistance in periodontal regeneration technique (SAI-PRT) in the treatment of periodontal intrabony defects: a case report with one-year follow-up. J Dent Res Dent Clin Dent Prospects. 2017;11(2):123–6. doi: 10.15171/joddd.2017.022. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

