Multiplex PCR system for the rapid diagnosis of respiratory virus infection: systematic review and meta-analysis
- PMID: 29208560
- PMCID: PMC7128951
- DOI: 10.1016/j.cmi.2017.11.018
Multiplex PCR system for the rapid diagnosis of respiratory virus infection: systematic review and meta-analysis
Abstract
Objectives: To provide a summary of evidence for the diagnostic accuracies of three multiplex PCR systems (mPCRs)-BioFire FilmArray RP (FilmArray), Nanosphere Verigene RV+ test (Verigene RV+) and Hologic Gen-Probe Prodesse assays-on the detection of viral respiratory infections.
Methods: A comprehensive search up to 1 July 2017 was conducted on Medline and Embase for studies that utilized FilmArray, Verigene RV+ and Prodesse for diagnosis of viral respiratory infections. A summary of diagnostic accuracies for the following five viruses were calculated: influenza A virus (FluA), influenza B virus, respiratory syncytial virus, human metapneumovirus and adenovirus. Hierarchical summary receiver operating curves were used for estimating the viral detection performance per assay.
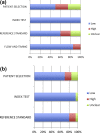
Results: Twenty studies of 5510 patient samples were eligible for analysis. Multiplex PCRs demonstrated high diagnostic accuracy, with area under the receiver operating characteristic curve (AUROC) equal to or more than 0.98 for all the above viruses except for adenovirus (AUROC 0.89). FilmArray, Verigene RV+ and ProFlu+ (the only Prodesse assay with enough data) demonstrated a summary sensitivity for FluA of 0.911 (95% confidence interval, 0.848-0.949), 0.949 (95% confidence interval, 0.882-0.979) and 0.954 (95% confidence interval, 0.871-0.985), respectively. The three mPCRs were comparable in terms of detection of FluA.
Conclusions: Point estimates calculated from eligible studies showed that the three mPCRs (FilmArray, Verigene RV+ and ProFlu+) are highly accurate and may provide important diagnostic information for early identification of respiratory virus infections. In patients with low pretest probability for FluA, these three mPCRs can predict a low possibility of infection and may justify withholding empirical antiviral treatments.
Keywords: Multiplex PCR; Point-of-care test; Respiratory virus infection.
Copyright © 2017 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Importance of accuracy of multiplex PCR systems for rapid diagnosis of respiratory virus infection.Clin Microbiol Infect. 2018 Oct;24(10):1033-1034. doi: 10.1016/j.cmi.2018.05.018. Epub 2018 Jun 2. Clin Microbiol Infect. 2018. PMID: 29870853 No abstract available.
References
-
- Williams B.G., Gouws E., Boschi-Pinto C., Bryce J., Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. - PubMed
-
- Marrie T.J., Durant H., Yates L. Community-acquired pneumonia requiring hospitalization: 5-year prospective study. Rev Infect Dis. 1989;11:586–599. - PubMed
-
- Byington C.L., Castillo H., Gerber K., Daly J.A., Brimley L.A., Adams S. The effect of rapid respiratory viral diagnostic testing on antibiotic use in a children’s hospital. Arch Pediatr Adolesc Med. 2002;156:1230–1234. - PubMed
-
- Henrickson K.J. Cost-effective use of rapid diagnostic techniques in the treatment and prevention of viral respiratory infections. Pediatr Ann. 2005;34:24–31. - PubMed
Further reading
-
- Sutton B.C., Maggert K., Rowell B., Etter R. Comparison of the focus diagnostics simplexa Flu A/B & RSV direct assay with the prodesse ProFlu+ assay for detection of influenza A virus (IAV), influenza B virus (IBV), and respiratory syncytial virus (RSV) in clinical specimens. J Mol Diagn. 2014;16:727–728.
-
- Espy M., Schneider S.K., Sloan L.M., Pritt B.S. Detection of influenza A, B and RSV in respiratory specimens by the proFlu+TM Kit and roche LC 480. J Mol Diagn. 2010;12:875.
-
- Irwin C., Matthews-Greer J., McRae K. Performance analysis of pandemic influenza detection methods. Am J Clin Pathol. 2012;138
-
- Buchan B., Anderson N., Jannetto P., Ledeboer N. Simultaneous detection of influenza A and its subtypes (H1, H3, 2009 H1N1), influenza B, and RSV A and B in respiratory specimens on an automated, random access, molecular platform. Clin Microbiol Infect. 2011;17:S3–S4.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

