Development of Anti-Virulence Approaches for Candidiasis via a Novel Series of Small-Molecule Inhibitors of Candida albicans Filamentation
- PMID: 29208749
- PMCID: PMC5717394
- DOI: 10.1128/mBio.01991-17
Development of Anti-Virulence Approaches for Candidiasis via a Novel Series of Small-Molecule Inhibitors of Candida albicans Filamentation
Abstract
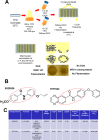
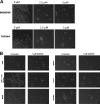
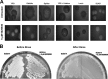
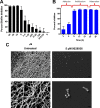
Candida albicans remains the main etiologic agent of candidiasis, the most common fungal infection and now the third most frequent infection in U.S. hospitals. The scarcity of antifungal agents and their limited efficacy contribute to the unacceptably high morbidity and mortality rates associated with these infections. The yeast-to-hypha transition represents the main virulence factor associated with the pathogenesis of C. albicans infections. In addition, filamentation is pivotal for robust biofilm development, which represents another major virulence factor for candidiasis and further complicates treatment. Targeting pathogenic mechanisms rather than growth represents an attractive yet clinically unexploited approach in the development of novel antifungal agents. Here, we performed large-scale phenotypic screening assays with 30,000 drug-like small-molecule compounds within ChemBridge's DIVERSet chemical library in order to identify small-molecule inhibitors of C. albicans filamentation, and our efforts led to the identification of a novel series of bioactive compounds with a common biaryl amide core structure. The leading compound of this series, N-[3-(allyloxy)-phenyl]-4-methoxybenzamide, was able to prevent filamentation under all liquid and solid medium conditions tested, suggesting that it impacts a common core component of the cellular machinery that mediates hypha formation under different environmental conditions. In addition to filamentation, this compound also inhibited C. albicans biofilm formation. This leading compound also demonstrated in vivo activity in clinically relevant murine models of invasive and oral candidiasis. Overall, our results indicate that compounds within this series represent promising candidates for the development of novel anti-virulence approaches to combat C. albicans infections.IMPORTANCE Since fungi are eukaryotes, there is a limited number of fungus-specific targets and, as a result, the antifungal arsenal is exceedingly small. Furthermore, the efficacy of antifungal treatment is compromised by toxicity and development of resistance. As a consequence, fungal infections carry high morbidity and mortality rates, and there is an urgent but unmet need for novel antifungal agents. One appealing strategy for antifungal drug development is to target pathogenetic mechanisms associated with infection. In Candida albicans, one of the most common pathogenic fungi, morphogenetic transitions between yeast cells and filamentous hyphae represent a key virulence factor associated with the ability of fungal cells to invade tissues, cause damage, and form biofilms. Here, we describe and characterize a novel small-molecule compound capable of inhibiting C. albicans filamentation both in vitro and in vivo; as such, this compound represents a leading candidate for the development of anti-virulence therapies against candidiasis.
Keywords: Candida albicans; anti-virulence factor; antifungal drugs; filamentation; large-scale phenotypic screening.
Copyright © 2017 Romo et al.
Figures

Similar articles
-
In Vitro Characterization of a Biaryl Amide Anti-virulence Compound Targeting Candida albicans Filamentation and Biofilm Formation.Front Cell Infect Microbiol. 2018 Jul 10;8:227. doi: 10.3389/fcimb.2018.00227. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30042929 Free PMC article.
-
Global Transcriptomic Analysis of the Candida albicans Response to Treatment with a Novel Inhibitor of Filamentation.mSphere. 2019 Sep 11;4(5):e00620-19. doi: 10.1128/mSphere.00620-19. mSphere. 2019. PMID: 31511371 Free PMC article.
-
Targeting Candida albicans filamentation for antifungal drug development.Virulence. 2017 Feb 17;8(2):150-158. doi: 10.1080/21505594.2016.1197444. Epub 2016 Jun 7. Virulence. 2017. PMID: 27268130 Free PMC article. Review.
-
Candidiasis drug discovery and development: new approaches targeting virulence for discovering and identifying new drugs.Expert Opin Drug Discov. 2013 Sep;8(9):1117-26. doi: 10.1517/17460441.2013.807245. Epub 2013 Jun 6. Expert Opin Drug Discov. 2013. PMID: 23738751 Free PMC article. Review.
-
High-content phenotypic screenings to identify inhibitors of Candida albicans biofilm formation and filamentation.Pathog Dis. 2014 Apr;70(3):423-31. doi: 10.1111/2049-632X.12161. Epub 2014 Mar 11. Pathog Dis. 2014. PMID: 24623598 Free PMC article.
Cited by
-
Synthetic Naphthofuranquinone Derivatives Are Effective in Eliminating Drug-Resistant Candida albicans in Hyphal, Biofilm, and Intracellular Forms: An Application for Skin-Infection Treatment.Front Microbiol. 2020 Aug 26;11:2053. doi: 10.3389/fmicb.2020.02053. eCollection 2020. Front Microbiol. 2020. PMID: 32983038 Free PMC article.
-
Bacterial-fungal interactions and their impact on microbial pathogenesis.Mol Ecol. 2023 May;32(10):2565-2581. doi: 10.1111/mec.16411. Epub 2022 Mar 14. Mol Ecol. 2023. PMID: 35231147 Free PMC article. Review.
-
New N-(oxazolylmethyl)-thiazolidinedione Active against Candida albicans Biofilm: Potential Als Proteins Inhibitors.Molecules. 2018 Oct 2;23(10):2522. doi: 10.3390/molecules23102522. Molecules. 2018. PMID: 30279343 Free PMC article.
-
A small molecule produced by Lactobacillus species blocks Candida albicans filamentation by inhibiting a DYRK1-family kinase.Nat Commun. 2021 Oct 22;12(1):6151. doi: 10.1038/s41467-021-26390-w. Nat Commun. 2021. PMID: 34686660 Free PMC article.
-
New Ground in Antifungal Discovery and Therapy for Invasive Fungal Infections: Innovations, Challenges, and Future Directions.J Fungi (Basel). 2024 Dec 15;10(12):871. doi: 10.3390/jof10120871. J Fungi (Basel). 2024. PMID: 39728367 Free PMC article. Review.
References
-
- Perea S, López-Ribot JL, Kirkpatrick WR, McAtee RK, Santillán RA, Martínez M, Calabrese D, Sanglard D, Patterson TF. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 45:2676–2684. doi:10.1128/AAC.45.10.2676-2684.2001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

