Thiamine and selected thiamine antivitamins - biological activity and methods of synthesis
- PMID: 29208764
- PMCID: PMC6435462
- DOI: 10.1042/BSR20171148
Thiamine and selected thiamine antivitamins - biological activity and methods of synthesis
Abstract
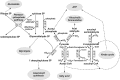
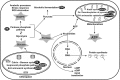
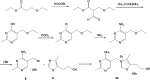
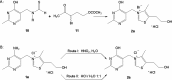
Thiamine plays a very important coenzymatic and non-coenzymatic role in the regulation of basic metabolism. Thiamine diphosphate is a coenzyme of many enzymes, most of which occur in prokaryotes. Pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes as well as transketolase are the examples of thiamine-dependent enzymes present in eukaryotes, including human. Therefore, thiamine is considered as drug or diet supplement which can support the treatment of many pathologies including neurodegenerative and vascular system diseases. On the other hand, thiamine antivitamins, which can interact with thiamine-dependent enzymes impeding their native functions, thiamine transport into the cells or a thiamine diphosphate synthesis, are good propose to drug design. The development of organic chemistry in the last century allowed the synthesis of various thiamine antimetabolites such as amprolium, pyrithiamine, oxythiamine, or 3-deazathiamine. Results of biochemical and theoretical chemistry research show that affinity to thiamine diphosphate-dependent enzymes of these synthetic molecules exceeds the affinity of native coenzyme. Therefore, some of them have already been used in the treatment of coccidiosis (amprolium), other are extensively studied as cytostatics in the treatment of cancer or fungal infections (oxythiamine and pyrithiamine). This review summarizes the current knowledge concerning the synthesis and mechanisms of action of selected thiamine antivitamins and indicates the potential of their practical use.
Keywords: 3-deazathiamine; amprolium; oxythiamine; pyrithiamine; thiamine.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures




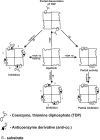

References
-
- Rapala-Kozik M. (2011) Vitamin B1 (Thiamine): a cofactor for enzymes involved in the main metabolic pathways and an environmental stress protectant. Adv. Bot. Res. 58, 37–91 10.1016/B978-0-12-386479-6.00004-4 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

