The orientation and stability of the GPCR-Arrestin complex in a lipid bilayer
- PMID: 29209002
- PMCID: PMC5716996
- DOI: 10.1038/s41598-017-17243-y
The orientation and stability of the GPCR-Arrestin complex in a lipid bilayer
Abstract
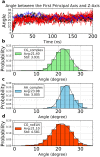
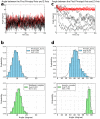
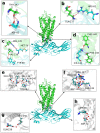
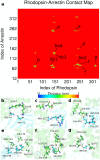
G protein-coupled receptors (GPCRs) constitute a large family of membrane proteins that plays a key role in transmembrane signal transduction and draw wide attention since it was discovered. Arrestin is a small family of proteins which can bind to GPCRs, block G protein interactions and redirect signaling to G-protein-independent pathways. The detailed mechanism of how arrestin interacts with GPCR remains elusive. Here, we conducted molecular dynamics simulations with coarse-grained (CG) and all-atom (AA) models to study the complex structure formed by arrestin and rhodopsin, a prototypical GPCR, in a POPC bilayer. Our results indicate that the formation of the complex has a significant impact on arrestin which is tightly anchored onto the bilayer surface, while has a minor effect on the orientation of rhodopsin in the lipid bilayer. The formation of the complex induces an internal change of conformation and flexibility in both rhodopsin and arrestin, mainly at the binding interface. Further investigation on the interaction interface identified the hydrogen bond network, especially the long-lived hydrogen bonds, and the key residues at the contact interface, which are responsible for stabilizing the complex. These results help us to better understand how rhodopsin interacts with arrestin on membranes, and thereby shed lights on arrestin-mediated signal transduction through GPCRs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

