Induced Pluripotent Stem Cell-Derived Neural Stem Cell Transplantations Reduced Behavioral Deficits and Ameliorated Neuropathological Changes in YAC128 Mouse Model of Huntington's Disease
- PMID: 29209158
- PMCID: PMC5701605
- DOI: 10.3389/fnins.2017.00628
Induced Pluripotent Stem Cell-Derived Neural Stem Cell Transplantations Reduced Behavioral Deficits and Ameliorated Neuropathological Changes in YAC128 Mouse Model of Huntington's Disease
Abstract
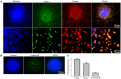
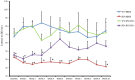
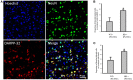
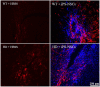
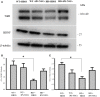
Huntington's disease (HD) is a genetic neurodegenerative disorder characterized by neuronal loss and motor dysfunction. Although there is no effective treatment, stem cell transplantation offers a promising therapeutic strategy, but the safety and efficacy of this approach needs to be optimized. The purpose of this study was to test the potential of intra-striatal transplantation of induced pluripotent stem cell-derived neural stem cells (iPS-NSCs) for treating HD. For this purpose, we developed mouse adenovirus-generated iPSCs, differentiated them into neural stem cells in vitro, labeled them with Hoechst, and transplanted them bilaterally into striata of 10-month old wild type (WT) and HD YAC128 mice. We assessed the efficiency of these transplanted iPS-NSCs to reduce motor deficits in YAC128 mice by testing them on an accelerating rotarod task at 1 day prior to transplantation, and then weekly for 10 weeks. Our results showed an amelioration of locomotor deficits in YAC128 mice that received iPS-NSC transplantations. Following testing, the mice were sacrificed, and their brains were analyzed using immunohistochemistry and Western blot (WB). The results from our histological examinations revealed no signs of tumors and evidence that many iPS-NSCs survived and differentiated into region-specific neurons (medium spiny neurons) in both WT and HD mice, as confirmed by co-labeling of Hoechst-labeled transplanted cells with NeuN and DARPP-32. Also, counts of Hoechst-labeled cells revealed that a higher proportion were co-labeled with DARPP-32 and NeuN in HD-, compared to WT- mice, suggesting a dissimilar differentiation pattern in HD mice. Whereas significant decreases were found in counts of NeuN- and DARPP-32-labeled cells, and for neuronal density measures in striata of HD vehicle controls, such decrements were not observed in the iPS-NSCs-transplanted-HD mice. WB analysis showed increase of BDNF and TrkB levels in striata of transplanted HD mice compared to HD vehicle controls. Collectively, our data suggest that iPS-NSCs may provide an effective option for neuronal replacement therapy in HD.
Keywords: Huntington's disease; YAC128; cell transplantations; iPS-NSCs; iPSCs; neural stem cells.
Figures


References
-
- Bernreuther C., Dihné M., Johann V., Schiefer J., Cui Y., Hargus G., et al. (2006). Neural cell adhesion molecule L1-transfected embryonic stem cells promote functional recovery after excitotoxic lesion of the mouse striatum. J. Neurosci. 26, 11532–11539. 10.1523/JNEUROSCI.2688-06.2006 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

