Molecular Mechanisms of Antipsychotic Drug-Induced Diabetes
- PMID: 29209160
- PMCID: PMC5702456
- DOI: 10.3389/fnins.2017.00643
Molecular Mechanisms of Antipsychotic Drug-Induced Diabetes
Abstract
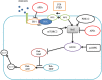
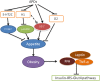
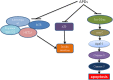
Antipsychotic drugs (APDs) are widely prescribed to control various mental disorders. As mental disorders are chronic diseases, these drugs are often used over a life-time. However, APDs can cause serious glucometabolic side-effects including type 2 diabetes and hyperglycaemic emergency, leading to medication non-compliance. At present, there is no effective approach to overcome these side-effects. Understanding the mechanisms for APD-induced diabetes should be helpful in prevention and treatment of these side-effects of APDs and thus improve the clinical outcomes of APDs. In this review, the potential mechanisms for APD-induced diabetes are summarized so that novel approaches can be considered to relieve APD-induced diabetes. APD-induced diabetes could be mediated by multiple mechanisms: (1) APDs can inhibit the insulin signaling pathway in the target cells such as muscle cells, hepatocytes and adipocytes to cause insulin resistance; (2) APD-induced obesity can result in high levels of free fatty acids (FFA) and inflammation, which can also cause insulin resistance. (3) APDs can cause direct damage to β-cells, leading to dysfunction and apoptosis of β-cells. A recent theory considers that both β-cell damage and insulin resistance are necessary factors for the development of diabetes. In high-fat diet-induced diabetes, the compensatory ability of β-cells is gradually damaged, while APDs cause direct β-cell damage, accounting for the severe form of APD-induced diabetes. Based on these mechanisms, effective prevention of APD-induced diabetes may need an integrated approach to combat various effects of APDs on multiple pathways.
Keywords: antipsychotics; apoptosis; diabetes mellitus; insulin resistance; metabolic disorders; obesity; pancreatic beta cell.
Figures
References
-
- Ader M., Kim S. P., Catalano K. J., Ionut V., Hucking K., Richey J. M., et al. . (2005). Metabolic dysregulation with atypical antipsychotics occurs in the absence of underlying disease a placebo-controlled study of olanzapine and risperidone in dogs. Diabetes 54, 862–871. 10.2337/diabetes.54.3.862 - DOI - PubMed
-
- Albaugh V. L., Judson J. G., She P., Lang C. H., Maresca K. P., Joyal J. L., et al. . (2011a). Olanzapine promotes fat accumulation in male rats by decreasing physical activity, repartitioning energy and increasing adipose tissue lipogenesis while impairing lipolysis. Mol. Psychiatry 16, 569–581. 10.1038/mp.2010.33 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

