Potential Mechanisms Underlying Inflammation-Enhanced Aminoglycoside-Induced Cochleotoxicity
- PMID: 29209174
- PMCID: PMC5702304
- DOI: 10.3389/fncel.2017.00362
Potential Mechanisms Underlying Inflammation-Enhanced Aminoglycoside-Induced Cochleotoxicity
Abstract
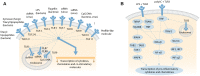
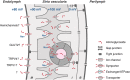
Aminoglycoside antibiotics remain widely used for urgent clinical treatment of life-threatening infections, despite the well-recognized risk of permanent hearing loss, i.e., cochleotoxicity. Recent studies show that aminoglycoside-induced cochleotoxicity is exacerbated by bacteriogenic-induced inflammation. This implies that those with severe bacterial infections (that induce systemic inflammation), and are treated with bactericidal aminoglycosides are at greater risk of drug-induced hearing loss than previously recognized. Incorporating this novel comorbid factor into cochleotoxicity risk prediction models will better predict which individuals are more predisposed to drug-induced hearing loss. Here, we review the cellular and/or signaling mechanisms by which host-mediated inflammatory responses to infection could enhance the trafficking of systemically administered aminoglycosides into the cochlea to enhance the degree of cochleotoxicity over that in healthy preclinical models. Once verified, these mechanisms will be potential targets for novel pharmacotherapeutics that reduce the risk of drug-induced hearing loss (and acute kidney damage) without compromising the life-saving bactericidal efficacy of aminoglycosides.
Keywords: aminoglycosides; bacteriogenic; gentamicin; infection; inflammation; ototoxicity; sepsis; virogenic.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

