Nonribosomal Peptides from Marine Microbes and Their Antimicrobial and Anticancer Potential
- PMID: 29209209
- PMCID: PMC5702503
- DOI: 10.3389/fphar.2017.00828
Nonribosomal Peptides from Marine Microbes and Their Antimicrobial and Anticancer Potential
Abstract
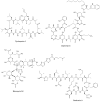
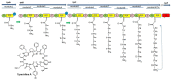
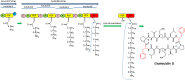
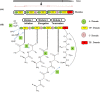
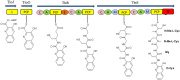
Marine environments are largely unexplored and can be a source of new molecules for the treatment of many diseases such as malaria, cancer, tuberculosis, HIV etc. The Marine environment is one of the untapped bioresource of getting pharmacologically active nonribosomal peptides (NRPs). Bioprospecting of marine microbes have achieved many remarkable milestones in pharmaceutics. Till date, more than 50% of drugs which are in clinical use belong to the nonribosomal peptide or mixed polyketide-nonribosomal peptide families of natural products isolated from marine bacteria, cyanobacteria and fungi. In recent years large numbers of nonribosomal have been discovered from marine microbes using multi-disciplinary approaches. The present review covers the NRPs discovered from marine microbes and their pharmacological potential along with role of genomics, proteomics and bioinformatics in discovery and development of nonribosomal peptides drugs.
Keywords: anticancer; antimicrobial; marine natural products; microbe derived-compounds; nonribosomal peptides.
Figures
References
-
- Andrianasolo E. H., Goeger D., Gerwick W. H. (2007). Mitsoamide: a cytotoxic linear lipopeptide from the Madagascar marine cyanobacterium Geitlerinema sp. Pure Appl. Chem. 79, 593–602. 10.1351/pac200779040593 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

