Critical Role of Hepatic Cyp450s in the Testis-Specific Toxicity of (5R)-5-Hydroxytriptolide in C57BL/6 Mice
- PMID: 29209210
- PMCID: PMC5702336
- DOI: 10.3389/fphar.2017.00832
Critical Role of Hepatic Cyp450s in the Testis-Specific Toxicity of (5R)-5-Hydroxytriptolide in C57BL/6 Mice
Abstract
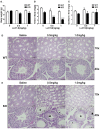
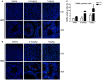
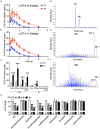
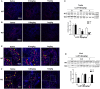
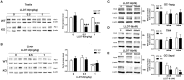
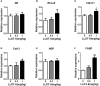
Low solubility, tissue accumulation, and toxicity are chief obstacles to developing triptolide derivatives, so a better understanding of the pharmacokinetics and toxicity of triptolide derivatives will help with these limitations. To address this, we studied pharmacokinetics and toxicity of (5R)-5-hydroxytriptolide (LLDT-8), a novel triptolide derivative immunosuppressant in a conditional knockout (KO) mouse model with liver-specific deletion of CYP450 reductase. Compared to wild type (WT) mice, after LLDT-8 treatment, KO mice suffered severe testicular toxicity (decreased testicular weight, spermatocytes apoptosis) unlike WT mice. Moreover, KO mice had greater LLDT-8 exposure as confirmed with elevated AUC and Cmax, increased drug half-life, and greater tissue distribution. γ-H2AX, a marker of meiosis process, its localization and protein level in testis showed a distinct meiosis block induced by LLDT-8. RNA polymerase II (Pol II), an essential factor for RNA storage and synapsis in spermatogenesis, decreased in testes of KO mice after LLDT-8 treatment. Germ-cell line based assays confirmed that LLDT-8 selectively inhibited Pol II in spermatocyte-like cells. Importantly, the analysis of androgen receptor (AR) related genes showed that LLDT-8 did not change AR-related signaling in testes. Thus, hepatic CYP450s were responsible for in vivo metabolism and clearance of LLDT-8 and aggravated testicular injury may be due to increased LLDT-8 exposure in testis and subsequent Pol II reduction.
Keywords: (5R)-5-hydroxytriptolide; RNA polymerase II; androgen receptor; cytochrome P450; functional knockout; testes; γ-H2AX.
Figures
Similar articles
-
Dephosphorylation of Tak1 at Ser412 greatly contributes to the spermatocyte-specific testis toxicity induced by (5R)-5-hydroxytriptolide in C57BL/6 mice.Toxicol Res (Camb). 2016 Jan 7;5(2):594-601. doi: 10.1039/c5tx00409h. eCollection 2016 Mar 1. Toxicol Res (Camb). 2016. PMID: 30090373 Free PMC article.
-
(5R)-5-hydroxytriptolide (LLDT-8), a novel triptolide analog mediates immunosuppressive effects in vitro and in vivo.Int Immunopharmacol. 2005 Dec;5(13-14):1895-903. doi: 10.1016/j.intimp.2005.06.009. Epub 2005 Jun 28. Int Immunopharmacol. 2005. PMID: 16275624
-
(5R)-5-hydroxytriptolide ameliorates liver lipid accumulation by suppressing lipid synthesis and promoting lipid oxidation in mice.Life Sci. 2019 Sep 1;232:116644. doi: 10.1016/j.lfs.2019.116644. Epub 2019 Jul 10. Life Sci. 2019. PMID: 31301418
-
Pharmacological activity and clinical progress of Triptolide and its derivatives LLDT-8, PG490-88Na, and Minnelide: a narrative review.Eur Rev Med Pharmacol Sci. 2023 Nov;27(21):10181-10203. doi: 10.26355/eurrev_202311_34294. Eur Rev Med Pharmacol Sci. 2023. PMID: 37975343 Review.
-
Nociceptin and meiosis during spermatogenesis in postnatal testes.Vitam Horm. 2015;97:167-86. doi: 10.1016/bs.vh.2014.10.003. Epub 2015 Jan 14. Vitam Horm. 2015. PMID: 25677772 Review.
Cited by
-
A Mechanistic Overview of Triptolide and Celastrol, Natural Products from Tripterygium wilfordii Hook F.Front Pharmacol. 2018 Feb 14;9:104. doi: 10.3389/fphar.2018.00104. eCollection 2018. Front Pharmacol. 2018. PMID: 29491837 Free PMC article. Review.
-
Therapeutic Effects of (5R)-5-Hydroxytriptolide on Fibroblast-Like Synoviocytes in Rheumatoid Arthritis via lncRNA WAKMAR2/miR-4478/E2F1/p53 Axis.Front Immunol. 2021 Feb 16;12:605616. doi: 10.3389/fimmu.2021.605616. eCollection 2021. Front Immunol. 2021. PMID: 33664742 Free PMC article.
-
The Yin and Yang of the Natural Product Triptolide and Its Interactions with XPB, an Essential Protein for Gene Expression and DNA Repair.Genes (Basel). 2024 Sep 30;15(10):1287. doi: 10.3390/genes15101287. Genes (Basel). 2024. PMID: 39457411 Free PMC article. Review.
References
-
- Du F., Liu T., Liu T., Wang Y., Wan Y., Xing J. (2011). Metabolite identification of triptolide by data-dependent accurate mass spectrometric analysis in combination with online hydrogen/deuterium exchange and multiple data-mining techniques. Rapid Commun. Mass Spectrom. 25, 3167–3177. 10.1002/rcm.5211 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

