Mesenchymal Stem Cells Induce Expression of CD73 in Human Monocytes In Vitro and in a Swine Model of Myocardial Infarction In Vivo
- PMID: 29209319
- PMCID: PMC5701925
- DOI: 10.3389/fimmu.2017.01577
Mesenchymal Stem Cells Induce Expression of CD73 in Human Monocytes In Vitro and in a Swine Model of Myocardial Infarction In Vivo
Abstract
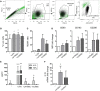
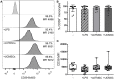
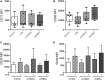
The ectoenzymes CD39 and CD73 regulate the purinergic signaling through the hydrolysis of adenosine triphosphate (ATP)/ADP to AMP and to adenosine (Ado), respectively. This shifts the pro-inflammatory milieu induced by extracellular ATP to the anti-inflammatory regulation by Ado. Mesenchymal stem cells (MSCs) have potent immunomodulatory capabilities, including monocyte modulation toward an anti-inflammatory phenotype aiding tissue repair. In vitro, we observed that human cardiac adipose tissue-derived MSCs (cATMSCs) and umbilical cord MSCs similarly polarize monocytes toward a regulatory M2 phenotype, which maintained the expression of CD39 and induced expression of CD73 in a cell contact dependent fashion, correlating with increased functional activity. In addition, the local treatment with porcine cATMSCs using an engineered bioactive graft promoted the in vivo CD73 expression on host monocytes in a swine model of myocardial infarction. Our results suggest the upregulation of ectonucleotidases on MSC-conditioned monocytes as an effective mechanism to amplify the long-lasting immunomodulatory and healing effects of MSCs delivery.
Keywords: CD73; adenosine; ectonucleotidase; immunomodulation; mesenchymal stem cell; myocardial infarction; purigernic signaling; regeneration.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

