Camelid Single-Domain Antibodies: Historical Perspective and Future Outlook
- PMID: 29209322
- PMCID: PMC5701970
- DOI: 10.3389/fimmu.2017.01589
Camelid Single-Domain Antibodies: Historical Perspective and Future Outlook
Abstract
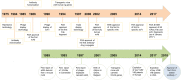
Tremendous effort has been expended over the past two and a half decades to understand many aspects of camelid heavy chain antibodies, from their biology, evolution, and immunogenetics to their potential applications in various fields of research and medicine. In this article, I present a historical perspective on the development of camelid single-domain antibodies (sdAbs or VHHs, also widely known as nanobodies) since their discovery and discuss the advantages and disadvantages of these unique molecules in various areas of research, industry, and medicine. Commercialization of camelid sdAbs exploded in 2001 with a flurry of patents issued to the Vrije Universiteit Brussel (VUB) and later taken on by the Vlaams Interuniversitair Instituut voor Biotechnologie (VIB) and, after 2002, the VIB-founded spin-off company, Ablynx. While entrepreneurial spirit has certainly catalyzed the exploration of nanobodies as marketable products, IP restrictions may be partially responsible for the relatively long time span between the discovery of these biomolecules and their entry into the pharmaceutical market. It is now anticipated that the first VHH-based antibody drug, Caplacizumab, a bivalent anti-vWF antibody for treating rare blood clotting disorders, may be approved and commercialized in 2018 or shortly thereafter. This elusive first approval, along with the expiry of key patents, may substantially alter the scientific and biomedical landscape surrounding camelid sdAbs and pave the way for their emergence as mainstream biotherapeutics.
Keywords: VHH; antibody engineering; camelid single-domain antibody; heavy chain antibody; nanobody; therapeutic antibody.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

