Comparative study of probiotic effects of Lactobacillus and Bifidobacteria strains on cholesterol levels, liver morphology and the gut microbiota in obese mice
- PMID: 29209439
- PMCID: PMC5700021
- DOI: 10.1007/s13167-017-0117-3
Comparative study of probiotic effects of Lactobacillus and Bifidobacteria strains on cholesterol levels, liver morphology and the gut microbiota in obese mice
Abstract
Background: Microbiome-modulating interventions are promising for treatment and prevention of metabolic syndrome. The number of probiotic strains demonstrated ability to decrease cholesterol level in vivo, however, it was poorly confirmed in a clinical setting. The aim was to study the effects of L. acidophilus IMV B-7279, L. casei IMV B-7280, B. animalіs VKL and B. animalіs VKB separately and in various compositions on the level of serum cholesterol, gut microbiota contents and liver morphology on a high-calorie-induced obesity model in BALB/c mice.
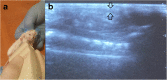
Materials and methods: We used for the study female BALB/c mice 6-8 weeks old (18-24 g). Experimental animals were fed by a fat-enriched diet (FED), and 8 experimental groups were formed (12 mice in each group) to test strains of probiotic bacteria L. delbrueckii subsp. bulgaricus IMV B-7281, L. casei IMV B-7280, B. animalіs VKL and B. animalіs VKB and compositions. We used ultrasound for in vivo assessment of the liver and visceral (mesenteric) fat size. In the blood serum of the obese mice, the level of cholesterol was estimated. The liver morphology and gut microbiota of obese mice were studied.
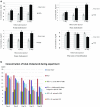
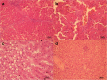
Results: We revealed that after treatment with all of the studied probiotic bacteria and compositions of B. animalis VKL/B. animalis VKB/L. casei IMV B-7280, the weight of obese mice decreased, and cholesterol and its fraction levels in serum were reduced. The size of the liver slightly decreased after treatment with L. delbrueckii subsp. bulgaricus IMV B-7281, B. аnimalis VKB or probiotic compositions; we observed reduction of the mesenteric fat size after injection of all these probiotic bacteria (separately) and probiotic compositions. We defined the strain-dependent effects on serum lipid profiles, liver morphology and the gut microbiota. The B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition effectively recovered the liver morphological structure of obese mice. The number of Lactobacillus spp., Bifidobacterium spp. and coliform bacteria increased, the number of staphylococci and streptococci reduced, and the number of microscopic fungi significantly decreased in the gut of obese mice after treatment with L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalis (separately) or their compositions.
Conclusion: L. casei IMV B-7280 (separately) and a composition of B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 are effective at decreasing the weight of obese mice, decreasing cholesterol level, restoring the liver morphology and beneficially modulating the gut microbiome in high-calorie-induced obesity.
Keywords: Bifidobacterium; Cholesterol; Gut microbiota; Lactobacillus; Liver; Metabolic syndrome; Mouse model: mesenteric fat; Obesity; Predictive preventive personalized medicine; Ultrasound.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Competing interests
Authors declare that they have no competing interests.
Figures



Similar articles
-
Can tailored nanoceria act as a prebiotic? Report on improved lipid profile and gut microbiota in obese mice.EPMA J. 2019 Oct 29;10(4):317-335. doi: 10.1007/s13167-019-00190-1. eCollection 2019 Dec. EPMA J. 2019. PMID: 31832109 Free PMC article.
-
Specific properties of probiotic strains: relevance and benefits for the host.EPMA J. 2018 Apr 13;9(2):205-223. doi: 10.1007/s13167-018-0132-z. eCollection 2018 Jun. EPMA J. 2018. PMID: 29896319 Free PMC article.
-
Probiotic strains of lactobacilli and bifidobacteria alter pro- and anti-inflammatory cytokines production in rats with monosodium glutamate-induced obesity.Fiziol Zh (1994). 2017;63(1):17-25. doi: 10.15407/fz63.01.017. Fiziol Zh (1994). 2017. PMID: 29975824
-
Selective and differential enumerations of Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium spp. in yoghurt--a review.Int J Food Microbiol. 2011 Oct 3;149(3):194-208. doi: 10.1016/j.ijfoodmicro.2011.07.008. Epub 2011 Jul 21. Int J Food Microbiol. 2011. PMID: 21807435 Review.
-
The Role of Bifidobacteria in Predictive and Preventive Medicine: A Focus on Eczema and Hypercholesterolemia.Microorganisms. 2021 Apr 14;9(4):836. doi: 10.3390/microorganisms9040836. Microorganisms. 2021. PMID: 33919907 Free PMC article. Review.
Cited by
-
Can tailored nanoceria act as a prebiotic? Report on improved lipid profile and gut microbiota in obese mice.EPMA J. 2019 Oct 29;10(4):317-335. doi: 10.1007/s13167-019-00190-1. eCollection 2019 Dec. EPMA J. 2019. PMID: 31832109 Free PMC article.
-
The causal relationship between gut microbiota and biliary tract cancer: comprehensive bidirectional Mendelian randomization analysis.Front Cell Infect Microbiol. 2024 Mar 15;14:1308742. doi: 10.3389/fcimb.2024.1308742. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38558852 Free PMC article.
-
Exploring the effects of probiotics on olanzapine-induced metabolic syndrome through the gut microbiota.Gut Pathog. 2024 Dec 21;16(1):77. doi: 10.1186/s13099-024-00664-2. Gut Pathog. 2024. PMID: 39709451 Free PMC article.
-
Potentially probiotic NPL 1334 strain of Enterococcus durans benefits rats with diet-induced hypercholesterolemia.BMC Biotechnol. 2025 Jan 17;25(1):7. doi: 10.1186/s12896-024-00943-5. BMC Biotechnol. 2025. PMID: 39825321 Free PMC article.
-
Symbiotic probiotic communities with multiple targets successfully combat obesity in high-fat-diet-fed mice.Gut Microbes. 2024 Jan-Dec;16(1):2420771. doi: 10.1080/19490976.2024.2420771. Epub 2024 Nov 3. Gut Microbes. 2024. PMID: 39488738 Free PMC article.
References
-
- WHO: Obesity and overweight: Fact sheet N. 311. http://www.who.int/mediacentre/factsheets/fs311/en/ Accessed 19 July 2017.
-
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375(9710):181–3. 10.1016/S0140-6736(09)61794-3. - PubMed
-
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21(1):1–6. doi: 10.1097/01.hco.0000200416.65370.a0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

