Milrinone in congenital diaphragmatic hernia - a randomized pilot trial: study protocol, review of literature and survey of current practices
- PMID: 29209510
- PMCID: PMC5704584
- DOI: 10.1186/s40748-017-0066-9
Milrinone in congenital diaphragmatic hernia - a randomized pilot trial: study protocol, review of literature and survey of current practices
Abstract
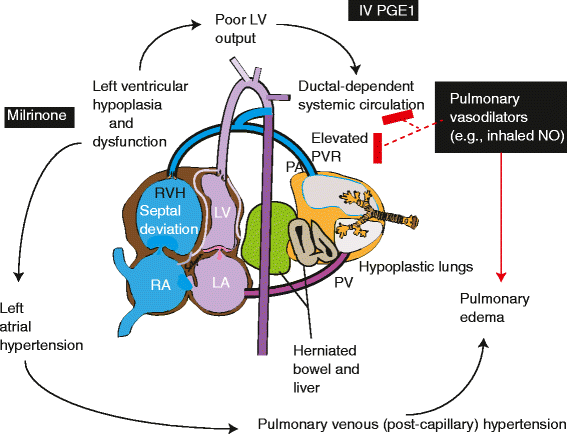
Background: Congenital diaphragmatic hernia (CDH) is commonly associated with pulmonary hypoplasia and pulmonary hypertension (PH). PH associated with CDH (CDH-PH) is frequently resistant to conventional pulmonary vasodilator therapy including inhaled nitric oxide (iNO) possibly due to right and left ventricular dysfunction. Milrinone is an intravenous inotrope and lusitrope with pulmonary vasodilator properties and has been shown anecdotally to improve oxygenation in PH. We developed this pilot study to determine if milrinone infusion would improve oxygenation in neonates ≥36 weeks postmenstrual age (PMA) with CDH.
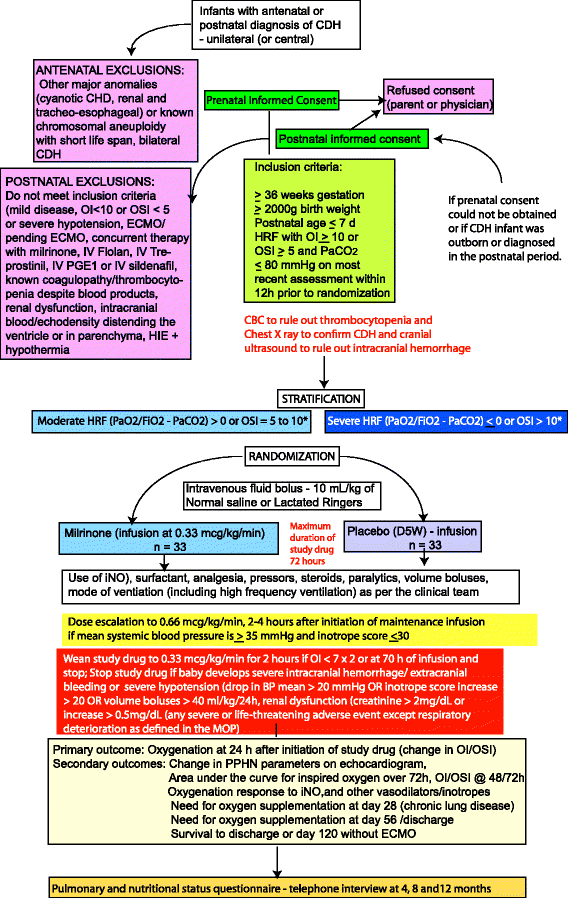
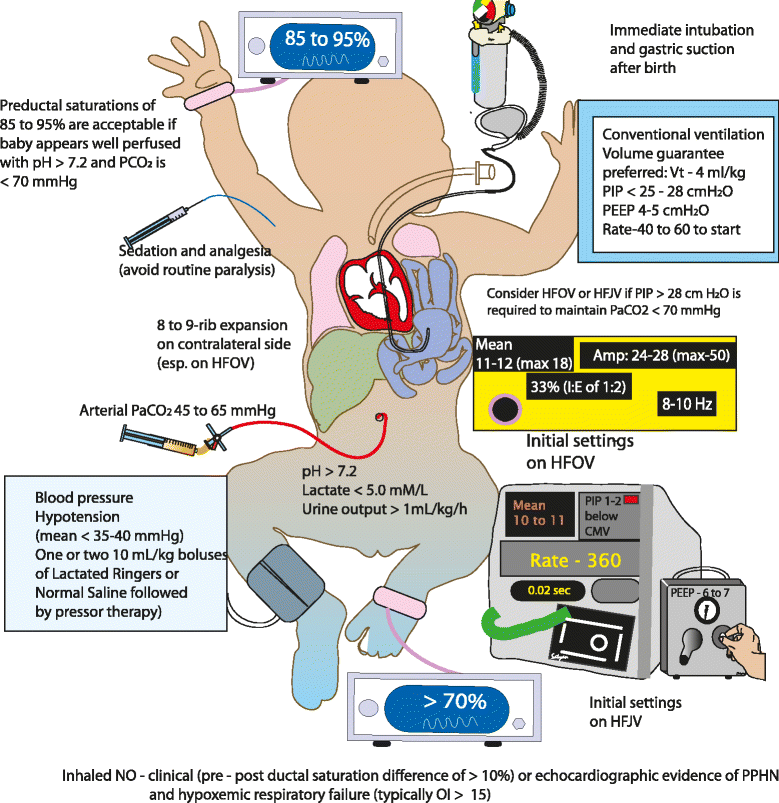
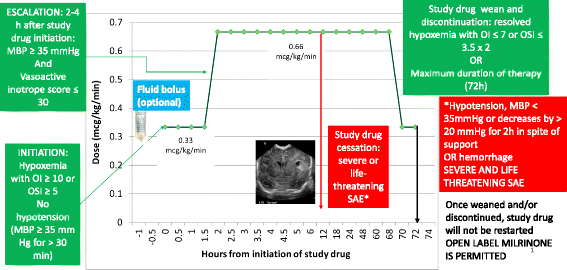
Methods/design: Data on pulmonary vasodilator management and outcome of CDH patients was collected from 18 university NICUs affiliated with the Neonatal Research Network (NRN) from 2011 to 2012. The proposed pilot will be a masked, placebo-controlled, multicenter, randomized trial of 66 infants with CDH with an oxygenation index (OI) ≥10 or oxygen saturation index (OSI) ≥5. The primary outcome is the oxygenation response, as determined by change in OI at 24 h after initiation of study drug. As secondary outcomes, we will determine oxygenation at 48 h and 72 h post-infusion, right ventricular pressures on echocardiogram and the incidence of systemic hypotension, arrhythmias, intracranial hemorrhage, survival without extracorporeal membrane oxygenation, and chronic lung disease (oxygen need at 28 days postnatal age). Finally, we will evaluate the pulmonary and nutritional status at 4, 8 and 12 months of age using a phone questionnaire.
Results: Three hundred thirty-seven infants with CDH were admitted to NRN NICUs in 2011 and 2012 of which 275 were ≥36 weeks PMA and were exposed to the following pulmonary vasodilators: iNO (39%), sildenafil (17%), milrinone (17%), inhaled epoprostenol (6%), intravenous epoprostenol (3%), and intravenous PGE1 (1%). ECMO was required in 36% of patients. Survival to discharge was 71%.
Discussion: CDH is an orphan disease with high mortality with few randomized trials evaluating postnatal management. Intravenous milrinone is a commonly used medication in neonatal/pediatric intensive care units and is currently used in 17% of patients with CDH within the NRN. This pilot study will provide data and enable further studies evaluating pulmonary vasodilator therapy in CDH.
Trial registration: ClinicalTrials.gov; NCT02951130; registered 14 October 2016.
Keywords: Extracorporeal membrane oxygenation; Oxygen; Persistent pulmonary hypertension; Phosphodiesterase; Pulmonary hypertension.
Conflict of interest statement
Ethics approval and consent to participate
No ethics approval or consent to participate was necessary for this manuscript.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Response to pulmonary vasodilators in infants with congenital diaphragmatic hernia.Pediatr Surg Int. 2018 Jul;34(7):735-742. doi: 10.1007/s00383-018-4286-5. Epub 2018 May 28. Pediatr Surg Int. 2018. PMID: 29808281
-
Management Practice and Mortality for Infants with Congenital Diaphragmatic Hernia.Am J Perinatol. 2015 Jul;32(9):887-94. doi: 10.1055/s-0035-1544949. Epub 2015 Feb 25. Am J Perinatol. 2015. PMID: 25715314 Free PMC article.
-
Management of pulmonary hypertension in infants with congenital diaphragmatic hernia.J Perinatol. 2016 Jun;36 Suppl 2:S28-31. doi: 10.1038/jp.2016.46. J Perinatol. 2016. PMID: 27225962 Review.
-
Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. The Neonatal Inhaled Nitric Oxide Study Group (NINOS).Pediatrics. 1997 Jun;99(6):838-45. doi: 10.1542/peds.99.6.838. Pediatrics. 1997. PMID: 9190553 Clinical Trial.
-
The Use of Inhaled Nitric Oxide in Congenital Diaphragmatic Hernia.Adv Neonatal Care. 2020 Dec;20(6):479-486. doi: 10.1097/ANC.0000000000000753. Adv Neonatal Care. 2020. PMID: 32384329 Review.
Cited by
-
Diagnosis, management and long term cardiovascular outcomes of phenotypic profiles in pulmonary hypertension associated with congenital diaphragmatic hernia.Front Pediatr. 2024 Mar 25;12:1356157. doi: 10.3389/fped.2024.1356157. eCollection 2024. Front Pediatr. 2024. PMID: 38590769 Free PMC article. Review.
-
Congenital diaphragmatic hernia.Nat Rev Dis Primers. 2022 Jun 1;8(1):37. doi: 10.1038/s41572-022-00362-w. Nat Rev Dis Primers. 2022. PMID: 35650272 Review.
-
Use of Prostaglandin E1 in the Management of Congenital Diaphragmatic Hernia-A Review.Front Pediatr. 2022 Jul 1;10:911588. doi: 10.3389/fped.2022.911588. eCollection 2022. Front Pediatr. 2022. PMID: 35844758 Free PMC article. Review.
-
The impact of a care bundle with an emphasis on hemodynamic assessment on the short-term outcomes in neonates with congenital diaphragmatic hernia.J Perinatol. 2024 Mar;44(3):348-353. doi: 10.1038/s41372-023-01807-0. Epub 2023 Nov 7. J Perinatol. 2024. PMID: 37935830
-
Optimal oxygenation and role of free radicals in PPHN.Free Radic Biol Med. 2019 Oct;142:97-106. doi: 10.1016/j.freeradbiomed.2019.04.001. Epub 2019 Apr 14. Free Radic Biol Med. 2019. PMID: 30995536 Free PMC article. Review.
References
-
- Keller RL. Management of the infant with congenital diaphragmatic hernia. In: Bancalari E, Polin RA, editors. The newborn lung. 2. Philadelphia: Elsevier Saunders; 2012. pp. 381–406.
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

