Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment
- PMID: 29209558
- PMCID: PMC5704598
- DOI: 10.1186/s40164-017-0091-4
Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment
Abstract
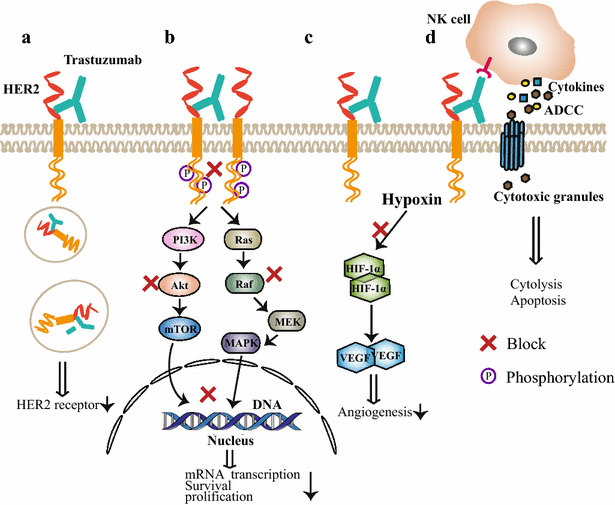
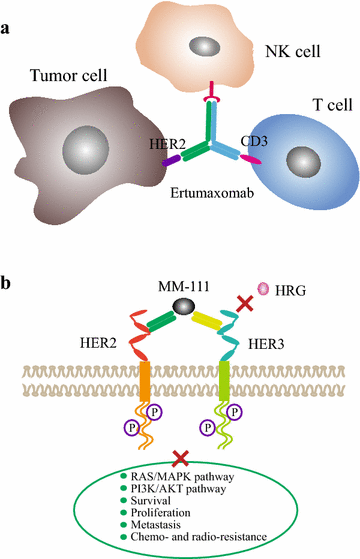
HER2-targeted immunotherapy consists of monoclonal antibodies (e.g. trastuzumab, pertuzumab), bispecific antibodies (e.g. MM-111, ertumaxomab) and activated T cells armed with anti-HER2 bispecific antibody (HER2Bi-aATC). Trastuzumab is a classic drug for the treatment of HER2 positive metastatic breast cancer. The combined application of pertuzumab, trastuzumab and paclitaxel has been suggested as a standard therapy for HER2 positive advanced breast cancer. The resistance to anti-HER2 antibody has resulted in disease progression. HER2-directed bispecific antibody may be a promising therapeutic approach for these patients. Ertumaxomab enhanced the interaction of immune effector cells and tumor cells. MM-111 simultaneously binds to HER2 and HER3 and blocks downstream signaling. Besides, HER2Bi-aATC is also an alternative therapeutic approach for HER2 positive cancers. In this review, we summarized the recent advancement of HER2-targeted monoclonal antibodies (trastuzumab, pertuzumab and T-DM1) and bispecific antibodies (MM-111, ertumaxomab and HER2Bi-aATC), especially focus on clinical trial results.
Keywords: Bispecific antibody; Ertumaxomab; HER2; HER2Bi-aATCs; MM-111; Pertuzumab; T-DM1; Trastuzumab.
Figures
References
-
- Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421(6924):756–760. - PubMed
-
- Jiang J, Dong L, Wang L, Wang L, Zhang J, Chen F, et al. HER2-targeted antibody drug conjugates for ovarian cancer therapy. Eur J Pharm Sci. 2016;93:274–286. - PubMed
-
- Yan M, Parker BA, Schwab R, Kurzrock R. HER2 aberrations in cancer: implications for therapy. Cancer Treat Rev. 2014;40(6):770–780. - PubMed
-
- Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5(1):63–69. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

