Silymarin Ameliorates Diabetes-Induced Proangiogenic Response in Brain Endothelial Cells through a GSK-3 β Inhibition-Induced Reduction of VEGF Release
- PMID: 29209632
- PMCID: PMC5676450
- DOI: 10.1155/2017/2537216
Silymarin Ameliorates Diabetes-Induced Proangiogenic Response in Brain Endothelial Cells through a GSK-3 β Inhibition-Induced Reduction of VEGF Release
Abstract
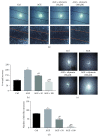
Diabetes mellitus (DM) is a major risk factor for cardiovascular disease. Additionally, it was found to induce a dysfunctional angiogenic response in the brain that was attributed to oxidative stress. Milk thistle seed extract (silymarin) has potent antioxidant properties, though its potential use in ameliorating diabetes-induced aberrant brain angiogenesis is unknown. Glycogen synthase kinase-3β is a regulator of angiogenesis that is upregulated by diabetes. Its involvement in diabetes-induced angiogenesis is unknown. To evaluate the potential of silymarin to ameliorate diabetes-induced aberrant angiogenesis, human brain endothelial cells (HBEC-5i) were treated with 50 μg/mL advanced glycation end (AGE) products in the presence or absence of silymarin (50, 100 μM). The angiogenic potential of HBEC-5i was evaluated in terms of migration and in vitro tube formation capacities. The involvement of GSK-3β was also evaluated. AGE significantly increased the migration and tube formation rates of HBEC-5i by about onefold (p = 0.0001). Silymarin reduced AGE-induced migration in a dose-dependent manner where 50 μM reduced migration by about 50%, whereas the 100 μM completely inhibited AGE-induced migration. Similarly, silymarin 50 μg/mL blunted AGE-induced tube formation (p = 0.001). This effect was mediated through a GSK-3β-dependent inhibition of VEGF release. In conclusion, silymarin inhibits AGE-induced aberrant angiogenesis in a GSK-3β-mediated inhibition of VEGF release.
Figures



References
-
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA, USA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

