The FDA's Expedited Programs and Clinical Development Times for Novel Therapeutics, 2012-2016
- PMID: 29209711
- PMCID: PMC5820715
- DOI: 10.1001/jama.2017.14896
The FDA's Expedited Programs and Clinical Development Times for Novel Therapeutics, 2012-2016
Abstract
This study uses the Drugs@FDA database to analyze clinical development times for drugs to treat serious diseases within the 4 FDA expedited programs intended to speed drug review and approval.
Conflict of interest statement
Figures
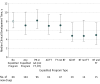
References
-
- US Food and Drug Administration Guidance for industry: expedited programs for serious conditions—drugs and biologics. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformat.... Accessed August 17, 2017.
-
- US Food and Drug Administration Novel drug approvals for 2016. https://www.fda.gov/drugs/developmentapprovalprocess/druginnovation/ucm4.... Accessed August 17, 2017.
-
- Kesselheim AS, Woloshin S, Eddings W, Franklin JM, Ross KM, Schwartz LM. Physicians’ knowledge about FDA approval standards and perceptions of the “breakthrough therapy” designation. JAMA. 2016;315(14):1516-1518. - PubMed
-
- Krishnamurti T, Woloshin S, Schwartz LM, Fischhoff B. A randomized trial testing US Food and Drug Administration “breakthrough” language. JAMA Intern Med. 2015;175(11):1856-1858. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

