Treatment of sporadic Burkitt lymphoma in adults, a retrospective comparison of four treatment regimens
- PMID: 29209924
- PMCID: PMC5754407
- DOI: 10.1007/s00277-017-3167-7
Treatment of sporadic Burkitt lymphoma in adults, a retrospective comparison of four treatment regimens
Abstract
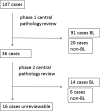
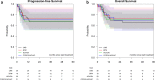
Burkitt lymphoma is an aggressive B cell malignancy accounting for 1-2% of all adult lymphomas. Treatment with dose-intensive, multi-agent chemotherapy is effective but associated with considerable toxicity. In this observational study, we compared real-world efficacy, toxicity, and costs of four frequently employed treatment strategies for Burkitt lymphoma: the Lymphome Malins B (LMB), the Berlin-Frankfurt-Münster (BFM), the HOVON, and the CODOX-M/IVAC regimens. We collected data from 147 adult patients treated in eight referral centers. Following central pathology assessment, 105 of these cases were accepted as Burkitt lymphoma, resulting in the following treatment groups: LMB 36 patients, BFM 19 patients, HOVON 29 patients, and CODOX-M/IVAC 21 patients (median age 39 years, range 14-74; mean duration of follow-up 47 months). There was no significant difference between age, sex ratio, disease stage, or percentage HIV-positive patients between the treatment groups. Five-year progression-free survival (69%, p = 0.966) and 5-year overall survival (69%, p = 0.981) were comparable for all treatment groups. Treatment-related toxicity was also comparable with only hepatotoxicity seen more frequently in the CODOX/M-IVAC group (p = 0.004). Costs were determined by the number of rituximab gifts and the number of inpatients days. Overall, CODOX-M/IVAC had the most beneficial profile with regards to costs, treatment duration, and percentage of patients completing planned treatment. We conclude that the four treatment protocols for Burkitt lymphoma yield nearly identical results with regards to efficacy and safety but differ in treatment duration and costs. These differences may help guide future choice of treatment.
Keywords: Burkitt lymphoma; Cost analysis; Drug therapy; Survival.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Figures
References
-
- Soussain C, Patte C, Ostronoff M, Delmer A, Rigalhuguet F, Cambier N, Leprise PY, Francois S, Conymakhoul P, Harousseau JL, Janvier M, Chauvenet L, Witz F, Pico J. Small noncleaved cell lymphoma and leukemia in adults-a retrospective study of 65 adults treated with the LMB pediatric protocols. Blood. 1995;85(3):664–674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

