Myelination and mTOR
- PMID: 29210103
- PMCID: PMC5836902
- DOI: 10.1002/glia.23273
Myelination and mTOR
Abstract
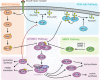
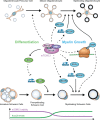
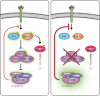
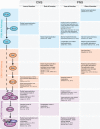
Myelinating cells surround axons to accelerate the propagation of action potentials, to support axonal health, and to refine neural circuits. Myelination is metabolically demanding and, consistent with this notion, mTORC1-a signaling hub coordinating cell metabolism-has been implicated as a key signal for myelination. Here, we will discuss metabolic aspects of myelination, illustrate the main metabolic processes regulated by mTORC1, and review advances on the role of mTORC1 in myelination of the central nervous system and the peripheral nervous system. Recent progress has revealed a complex role of mTORC1 in myelinating cells that includes, besides positive regulation of myelin growth, additional critical functions in the stages preceding active myelination. Based on the available evidence, we will also highlight potential nonoverlapping roles between mTORC1 and its known main upstream pathways PI3K-Akt, Mek-Erk1/2, and AMPK in myelinating cells. Finally, we will discuss signals that are already known or hypothesized to be responsible for the regulation of mTORC1 activity in myelinating cells.
Keywords: Schwann cell; mTOR; metabolism; myelin; oligodendrocyte.
© 2017 The Authors GLIA Published by Wiley Periodicals, Inc.
Figures

References
-
- Ando, S. , Tanaka, Y. , Toyoda, Y. , & Kon, K. (2003). Turnover of myelin lipids in aging brain. Neurochemical Research, 28, 5–13. - PubMed
-
- Anjum, R. , & Blenis, J. (2008). The RSK family of kinases: Emerging roles in cellular signalling. Nature Reviews Molecular Cell Biology, 9, 747–758. - PubMed
-
- Barres, B. A. , & Raff, M. C. (1994). Control of oligodendrocyte number in the developing rat optic nerve. Neuron, 12, 935–942. - PubMed
-
- Baumann, N. , & Pham‐Dinh, D. (2001). Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological Reviews, 81, 871–927. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

