Meta-Analysis of Fecal Microbiota and Metabolites in Experimental Colitic Mice during the Inflammatory and Healing Phases
- PMID: 29211010
- PMCID: PMC5748779
- DOI: 10.3390/nu9121329
Meta-Analysis of Fecal Microbiota and Metabolites in Experimental Colitic Mice during the Inflammatory and Healing Phases
Abstract
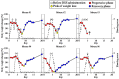
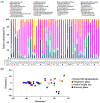
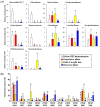
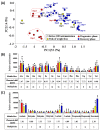
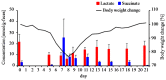
The imbalance of gut microbiota is known to be associated with inflammatory bowel disease, but it remains unknown whether dysbiosis is a cause or consequence of chronic gut inflammation. In order to investigate the effects of gut inflammation on microbiota and metabolome, the sequential changes in gut microbiota and metabolites from the onset of colitis to the recovery in dextran sulfate sodium-induced colitic mice were characterized by using meta 16S rRNA sequencing and proton nuclear magnetic resonance (¹H-NMR) analysis. Mice in the colitis progression phase showed the transient expansions of two bacterial families including Bacteroidaceae and Enterobacteriaceae and the depletion of major gut commensal bacteria belonging to the uncultured Bacteroidales family S24-7, Rikenellaceae, Lachnospiraceae, and Ruminococcaceae. After the initiation of the recovery, commensal Lactobacillus members promptly predominated in gut while other normally abundant bacteria excluding the Erysipelotrichaceae remained diminished. Furthermore, ¹H-NMR analysis revealed characteristic fluctuations in fecal levels of organic acids (lactate and succinate) associated with the disease states. In conclusion, acute intestinal inflammation is a perturbation factor of gut microbiota but alters the intestinal environments suitable for Lactobacillus members.
Keywords: 1H-NMR analysis; Lactobacillus; dysbiosis; experimental colitic mice; gut microbiota; inflammatory bowel disease; meta 16S rRNA analysis; metabolome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Contractor N.V., Bassiri H., Reya T., Park A.Y., Baumgart D.C., Wasik M.A., Emerson S.G., Carding S.R. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J. Immunol. 1998;160:385–394. - PubMed
-
- Song F., Ito K., Denning T.L., Kuninger D., Papaconstantinou J., Gourley W., Klimpel G., Balish E., Hokanson J., Ernst P.B. Expression of the neutrophil chemokine KC in the colon of mice with enterocolitis and by intestinal epithelial cell lines: Effects of flora and proinflammatory cytokines. J. Immunol. 1999;162:2275–2280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

