In Vitro and In Vivo Antioxidant and Anti-Hyperglycemic Activities of Moroccan Oat Cultivars
- PMID: 29211033
- PMCID: PMC5745512
- DOI: 10.3390/antiox6040102
In Vitro and In Vivo Antioxidant and Anti-Hyperglycemic Activities of Moroccan Oat Cultivars
Abstract
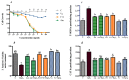
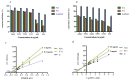
Improvement of oat lines via introgression is an important process for food biochemical functionality. This work aims to evaluate the protective effect of phenolic compounds from hybrid Oat line (F11-5) and its parent (Amlal) on hyperglycemia-induced oxidative stress and to establish the possible mechanisms of antidiabetic activity by digestive enzyme inhibition. Eight phenolic acids were quantified in our samples including ferulic, p-hydroxybenzoic, caffeic, salicylic, syringic, sinapic, p-coumaric and chlorogenic acids. The Oat extract (2000 mg/kg) ameliorated the glucose tolerance, decreased Fasting Blood Glucose (FBG) and oxidative stress markers, including Superoxide dismutase (SOD), Catalase (CAT), Glutathione peroxidase (GPx), Glutathione (GSH) and Malondialdehyde (MDA) in rat liver and kidney. Furthermore, Metformin and Oat intake prevented anxiety, hypercholesterolemia and atherosclerosis in diabetic rats. In vivo anti-hyperglycemic effect of Oat extracts has been confirmed by their inhibitory activities on α-amylase (723.91 μg/mL and 1027.14 μg/mL) and α-glucosidase (1548.12 μg/mL & 1803.52 μg/mL) enzymes by mean of a mixed inhibition.
Keywords: anti-hyperglycemic; digestive enzyme; hybrid Oat; streptozotocin-nicotinamide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

