Luminescence-Based Optical Sensors Fabricated by Means of the Layer-by-Layer Nano-Assembly Technique
- PMID: 29211050
- PMCID: PMC5751518
- DOI: 10.3390/s17122826
Luminescence-Based Optical Sensors Fabricated by Means of the Layer-by-Layer Nano-Assembly Technique
Abstract
Luminescence-based sensing applications range from agriculture to biology, including medicine and environmental care, which indicates the importance of this technique as a detection tool. Luminescent optical sensors are required to be highly stable, sensitive, and selective, three crucial features that can be achieved by fabricating them by means of the layer-by-layer nano-assembly technique. This method permits us to tailor the sensors' properties at the nanometer scale, avoiding luminophore aggregation and, hence, self-quenching, promoting the diffusion of the target analytes, and building a barrier against the undesired molecules. These characteristics give rise to the fabrication of custom-made sensors for each particular application.
Keywords: chemical sensing; layer-by-layer nano-assembly technique; nanostructured materials; photoluminescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







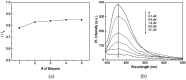









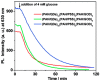
References
-
- Sun C., Chen Y., Zhang G., Wang F., Liu G., Ding J. Multipoint Remote Methane Measurement System Based on Spectrum Absorption and Reflective TDM. IEEE Photonics Technol. Lett. 2016;28:2487–2490. doi: 10.1109/LPT.2016.2601625. - DOI
-
- Tan C.H., Tan S.T., Lee H.B., Ginting R.T., Oleiwi H.F., Yap C.C., Jumali M.H.H., Yahaya M. Automated room temperature optical absorbance CO sensor based on In-doped ZnO nanorod. Sens. Actuators B Chem. 2017;248:140–152. doi: 10.1016/j.snb.2017.02.161. - DOI
-
- Del Villar I., Arregui F.J., Zamarreño C.R., Corres J.M., Bariain C., Goicoechea J., Elosua C., Hernaez M., Rivero P.J., Socorro A.B., et al. Optical sensors based on lossy-mode resonances. Sens. Actuators B Chem. 2017;240:174–185. doi: 10.1016/j.snb.2016.08.126. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

