Dose, schedule, safety, and efficacy of guadecitabine in relapsed or refractory acute myeloid leukemia
- PMID: 29211308
- PMCID: PMC5814873
- DOI: 10.1002/cncr.31138
Dose, schedule, safety, and efficacy of guadecitabine in relapsed or refractory acute myeloid leukemia
Abstract
Background: Outcomes for patients with relapsed or refractory acute myeloid leukemia (AML) are poor. Guadecitabine, a next-generation hypomethylating agent, could be useful in treating such patients.
Methods: In this multicenter, open-label, phase 2 dose-expansion study, AML patients from 10 North American medical centers were first randomized (1:1) to receive subcutaneous guadecitabine at 60 or 90 mg/m2 on 5 consecutive days in each 28-day cycle (5-day regimen). Subsequently, another cohort was treated for 10 days with 60 mg/m2 (10-day regimen).
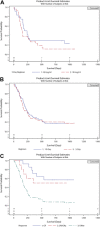
Results: Between June 15, 2012, and August 19, 2013, 108 patients with previously treated AML consented to enroll in the study, and 103 of these patients were treated; 5 patients did not receive the study treatment. A total of 103 patients were included in the safety and efficacy analyses (24 and 26 patients who were randomly assigned to 60 and 90 mg/m2 /d, respectively [5-day regimen] and 53 patients who were assigned to 60 mg/m2 /d [10-day regimen]). The 90 mg/m2 dose showed no benefit in clinical outcomes in comparison with 60 mg/m2 in the randomized cohort. Composite complete response (CRc) and complete response (CR) rates were higher with the 10-day regimen versus the 5-day regimen (CRc, 30.2% vs 16.0%; P = .1061; CR, 18.9% vs 8%; P = .15). Adverse events (grade ≥ 3) were mainly hematologic, with a higher incidence on the 10-day regimen. Early all-cause mortality was low and similar between regimens. Twenty patients (8 on the 5-day regimen and 12 on the 10-day regimen) were bridged to hematopoietic cell transplantation.
Conclusions: Guadecitabine has promising clinical activity and an acceptable safety profile and thus warrants further development in this population. Cancer 2018;124:325-34. © 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the Creative Commons Attribution NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Keywords: SGI-110; acute myeloid leukemia (AML); guadecitabine; refractory; relapsed.
© 2017 American Cancer Society.
Figures


Comment in
-
"Epigenetic" modification as therapy for acute myeloid leukemia.Cancer. 2018 Jan 15;124(2):242-244. doi: 10.1002/cncr.31137. Epub 2017 Dec 6. Cancer. 2018. PMID: 29211296 Free PMC article. No abstract available.
References
-
- Roboz G, Rosenbalt T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2014;32:1919‐1926. - PubMed
-
- Issa JP, Garcia‐Manero G, Giles FJ, et al. Phase 1 study of low‐dose prolonged exposure schedules of the hypomethylating agent 5‐aza‐2′‐deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103:1635‐1640. - PubMed
-
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, #open |label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low‐dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670‐2677. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

