Pharmacist-based antihypertensive medication review and assignment of morning versus evening dosing of once-daily antihypertensive medications: A pilot study to assess feasibility and efficacy in chronic kidney disease patients
- PMID: 29211509
- PMCID: PMC6098414
- DOI: 10.1080/10641963.2017.1411493
Pharmacist-based antihypertensive medication review and assignment of morning versus evening dosing of once-daily antihypertensive medications: A pilot study to assess feasibility and efficacy in chronic kidney disease patients
Abstract
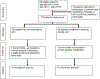
Evening dosing of antihypertensive medications lowers nighttime blood pressure, and in one large randomized trial, it reduced the risk for cardiovascular outcomes. However, the feasibility of nighttime dosing in routine clinical practice is unknown. The purpose of this pilot study was to evaluate the effect of a brief pharmacist intervention to assign patients to take antihypertensive medications at specific times of the day. In this pilot, randomized controlled trial, 79 patients with moderate to severe chronic kidney disease (CKD) taking one or more antihypertensive medications once daily were randomized to take one once-daily antihypertensive either in the morning or in the evening. A total of 79 patients were randomized (39 to morning dosing, 40 to evening dosing). Average (SD) age was 56.5 (14) years, 68% were male, and average (SD) estimated glomerular filtration rate (eGFR) was 36.6 (8.9) mL/min/1.73m2. Adherence, defined as taking the once-daily medication at the time indicated six or seven times in the last 7 days and not taking it at any other time during the day, was 91% in the morning arm and 95% in the evening arm (p = 0.57). This pilot demonstrates the feasibility and efficacy of a pharmacist-physician collaborative to assign once-daily antihypertensive medications to either morning or evening dosing.
Keywords: Hypertension; adherence; chronic kidney disease; chronotherapy.
Conflict of interest statement
The authors have no conflicts of interests, financial or otherwise, to disclose.
References
-
- James PA, Oparil S, Carter BL, et al. 2014. evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA February 05 2014;311(5):507–520. - PubMed
-
- Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension January 2011;57(1):3–10. - PubMed
-
- Minutolo R, Gabbai FB, Borrelli S, et al. Changing the timing of antihypertensive therapy to reduce nocturnal blood pressure in CKD: an 8-week uncontrolled trial. Am J Kidney Dis December 2007;50(6):908–917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous