Pharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis
- PMID: 29211671
- PMCID: PMC5763501
- DOI: 10.1056/NEJMoa1615066
Pharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis
Abstract
Background: The post-thrombotic syndrome frequently develops in patients with proximal deep-vein thrombosis despite treatment with anticoagulant therapy. Pharmacomechanical catheter-directed thrombolysis (hereafter "pharmacomechanical thrombolysis") rapidly removes thrombus and is hypothesized to reduce the risk of the post-thrombotic syndrome.
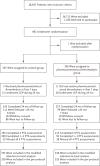
Methods: We randomly assigned 692 patients with acute proximal deep-vein thrombosis to receive either anticoagulation alone (control group) or anticoagulation plus pharmacomechanical thrombolysis (catheter-mediated or device-mediated intrathrombus delivery of recombinant tissue plasminogen activator and thrombus aspiration or maceration, with or without stenting). The primary outcome was development of the post-thrombotic syndrome between 6 and 24 months of follow-up.
Results: Between 6 and 24 months, there was no significant between-group difference in the percentage of patients with the post-thrombotic syndrome (47% in the pharmacomechanical-thrombolysis group and 48% in the control group; risk ratio, 0.96; 95% confidence interval [CI], 0.82 to 1.11; P=0.56). Pharmacomechanical thrombolysis led to more major bleeding events within 10 days (1.7% vs. 0.3% of patients, P=0.049), but no significant difference in recurrent venous thromboembolism was seen over the 24-month follow-up period (12% in the pharmacomechanical-thrombolysis group and 8% in the control group, P=0.09). Moderate-to-severe post-thrombotic syndrome occurred in 18% of patients in the pharmacomechanical-thrombolysis group versus 24% of those in the control group (risk ratio, 0.73; 95% CI, 0.54 to 0.98; P=0.04). Severity scores for the post-thrombotic syndrome were lower in the pharmacomechanical-thrombolysis group than in the control group at 6, 12, 18, and 24 months of follow-up (P<0.01 for the comparison of the Villalta scores at each time point), but the improvement in quality of life from baseline to 24 months did not differ significantly between the treatment groups.
Conclusions: Among patients with acute proximal deep-vein thrombosis, the addition of pharmacomechanical catheter-directed thrombolysis to anticoagulation did not result in a lower risk of the post-thrombotic syndrome but did result in a higher risk of major bleeding. (Funded by the National Heart, Lung, and Blood Institute and others; ATTRACT ClinicalTrials.gov number, NCT00790335 .).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
The ATTRACTiveness of catheter-directed thrombolysis.J Vasc Surg Venous Lymphat Disord. 2018 May;6(3):303. doi: 10.1016/j.jvsv.2018.02.002. J Vasc Surg Venous Lymphat Disord. 2018. PMID: 29661361 No abstract available.
-
Pharmacomechanical Therapy for Deep-Vein Thrombosis.N Engl J Med. 2018 May 3;378(18):1752. doi: 10.1056/NEJMc1802596. N Engl J Med. 2018. PMID: 29722517 No abstract available.
-
The ATTRACT Trial Becomes More Attractive.Eur J Vasc Endovasc Surg. 2019 Jun;57(6):755-756. doi: 10.1016/j.ejvs.2019.03.017. Epub 2019 Apr 11. Eur J Vasc Endovasc Surg. 2019. PMID: 30982730 No abstract available.
-
Pulmonary Embolism: Controversies in Therapeutic Management.Am J Respir Crit Care Med. 2020 Jan 15;201(2):240-242. doi: 10.1164/rccm.201812-2357RR. Am J Respir Crit Care Med. 2020. PMID: 31794251 No abstract available.
References
-
- Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249–56. - PubMed
-
- Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the post-thrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698–707. - PubMed
-
- Enden T, Haig Y, Kløw NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379:31–8. - PubMed
-
- Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880–8. - PubMed
-
- Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–12. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
