Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant
- PMID: 29211678
- PMCID: PMC6029626
- DOI: 10.1056/NEJMoa1708538
Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant
Abstract
Background: The prevention of bleeding with adequately sustained levels of clotting factor, after a single therapeutic intervention and without the need for further medical intervention, represents an important goal in the treatment of hemophilia.
Methods: We infused a single-stranded adeno-associated viral (AAV) vector consisting of a bioengineered capsid, liver-specific promoter and factor IX Padua (factor IX-R338L) transgene at a dose of 5×1011 vector genomes per kilogram of body weight in 10 men with hemophilia B who had factor IX coagulant activity of 2% or less of the normal value. Laboratory values, bleeding frequency, and consumption of factor IX concentrate were prospectively evaluated after vector infusion and were compared with baseline values.
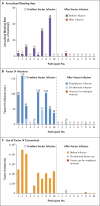
Results: No serious adverse events occurred during or after vector infusion. Vector-derived factor IX coagulant activity was sustained in all the participants, with a mean (±SD) steady-state factor IX coagulant activity of 33.7±18.5% (range, 14 to 81). On cumulative follow-up of 492 weeks among all the participants (range of follow-up in individual participants, 28 to 78 weeks), the annualized bleeding rate was significantly reduced (mean rate, 11.1 events per year [range, 0 to 48] before vector administration vs. 0.4 events per year [range, 0 to 4] after administration; P=0.02), as was factor use (mean dose, 2908 IU per kilogram [range, 0 to 8090] before vector administration vs. 49.3 IU per kilogram [range, 0 to 376] after administration; P=0.004). A total of 8 of 10 participants did not use factor, and 9 of 10 did not have bleeds after vector administration. An asymptomatic increase in liver-enzyme levels developed in 2 participants and resolved with short-term prednisone treatment. One participant, who had substantial, advanced arthropathy at baseline, administered factor for bleeding but overall used 91% less factor than before vector infusion.
Conclusions: We found sustained therapeutic expression of factor IX coagulant activity after gene transfer in 10 participants with hemophilia who received the same vector dose. Transgene-derived factor IX coagulant activity enabled the termination of baseline prophylaxis and the near elimination of bleeding and factor use. (Funded by Spark Therapeutics and Pfizer; ClinicalTrials.gov number, NCT02484092 .).
Figures
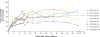

Comment in
-
Closing In on Treatment for Hemophilia B.N Engl J Med. 2017 Dec 7;377(23):2274-2275. doi: 10.1056/NEJMe1713735. N Engl J Med. 2017. PMID: 29211662 No abstract available.
-
A Cure for Hemophilia within Reach.N Engl J Med. 2017 Dec 28;377(26):2592-2593. doi: 10.1056/NEJMe1713888. Epub 2017 Dec 9. N Engl J Med. 2017. PMID: 29224412 No abstract available.
-
FIX It in One Go: Enhanced Factor IX Gene Therapy for Hemophilia B.Cell. 2017 Dec 14;171(7):1478-1480. doi: 10.1016/j.cell.2017.11.049. Cell. 2017. PMID: 29245009
-
Hyperactive Factor IX Padua: A Game-Changer for Hemophilia Gene Therapy.Mol Ther. 2018 Jan 3;26(1):14-16. doi: 10.1016/j.ymthe.2017.12.007. Epub 2017 Dec 21. Mol Ther. 2018. PMID: 29274719 Free PMC article. No abstract available.
References
-
- Mannucci PM, Tuddenham EGD. The hemophilias — from royal genes to gene therapy. N Engl J Med. 2001;344:1773–9. - PubMed
-
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. - PubMed
-
- Den Uijl IE, Mauser Bunschoten EP, Roosendaal G, et al. Clinical severity of haemophilia A: does the classification of the 1950s still stand? Haemophilia. 2011;17:849–53. - PubMed
-
- Roth DA, Tawa NE, Jr, O’Brien JM, Treco DA, Selden RF. Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N Engl J Med. 2001;344:1735–42. - PubMed
-
- Powell JS, Ragni MV, White GC, II, et al. Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood. 2003;102:2038–45. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
