Targeting Glutamine Metabolism for Cancer Treatment
- PMID: 29212303
- PMCID: PMC5746034
- DOI: 10.4062/biomolther.2017.178
Targeting Glutamine Metabolism for Cancer Treatment
Abstract
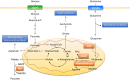
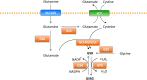
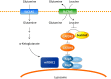
Rapidly proliferating cancer cells require energy and cellular building blocks for their growth and ability to maintain redox balance. Many studies have focused on understanding how cancer cells adapt their nutrient metabolism to meet the high demand of anabolism required for proliferation and maintaining redox balance. Glutamine, the most abundant amino acid in plasma, is a well-known nutrient used by cancer cells to increase proliferation as well as survival under metabolic stress conditions. In this review, we provide an overview of the role of glutamine metabolism in cancer cell survival and growth and highlight the mechanisms by which glutamine metabolism affects cancer cell signaling. Furthermore, we summarize the potential therapeutic approaches of targeting glutamine metabolism for the treatment of numerous types of cancer.
Keywords: Anaplerosis; Cancer; Glutamine; Redox homeostasis.
Figures
References
-
- Baenke F, Chaneton B, Smith M, Van Den Broek N, Hogan K, Tang H, Viros A, Martin M, Galbraith L, Girotti MR, Dhomen N, Gottlieb E, Marais R. Resistance to BRAF inhibitors induces glutamine dependency in melanoma cells. Mol Oncol. 2016;10:73–84. doi: 10.1016/j.molonc.2015.08.003. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

