Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodulation and osteogenesis
- PMID: 29212557
- PMCID: PMC5719931
- DOI: 10.1186/s13287-017-0730-z
Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodulation and osteogenesis
Abstract
Background: Mesenchymal stem cells (MSCs) are capable of immunomodulation and tissue regeneration, highlighting their potential translational application for treating inflammatory bone disorders. MSC-mediated immunomodulation is regulated by proinflammatory cytokines and pathogen-associated molecular patterns such as lipopolysaccharide (LPS). Previous studies showed that MSCs exposed to interferon gamma (IFN-γ) and the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) synergistically suppressed T-cell activation.
Methods: In the current study, we developed a novel preconditioning strategy for MSCs using LPS plus TNF-α to optimize the immunomodulating ability of MSCs on macrophage polarization.
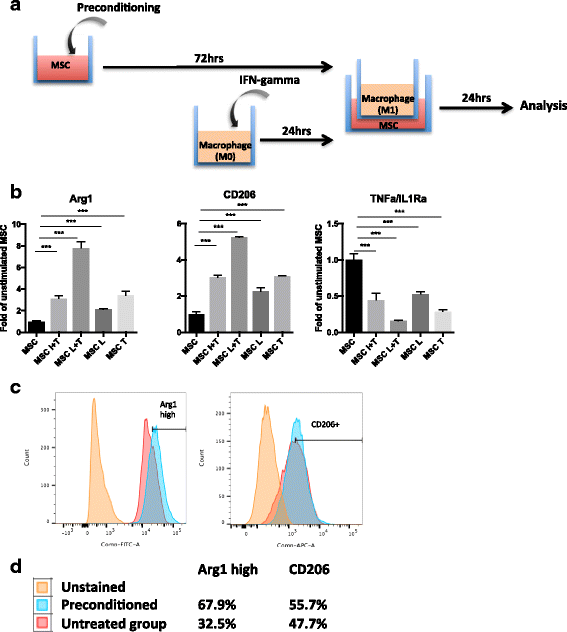
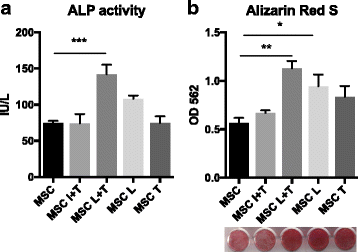
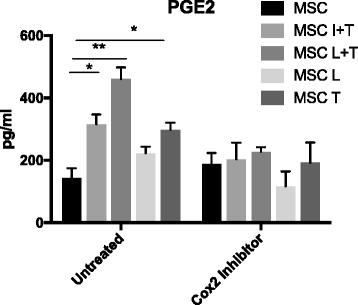
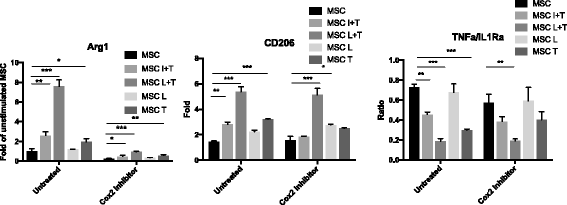
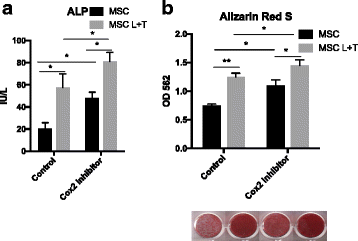
Results: Preconditioned MSCs enhanced anti-inflammatory M2 macrophage marker expression (Arginase 1 and CD206) and decreased inflammatory M1 macrophage marker (TNF-α/IL-1Ra) expression using an in-vitro coculture model. Immunomodulation of MSCs on macrophages was significantly increased compared to the combination of IFN-γ plus TNF-α or single treatment controls. Increased osteogenic differentiation including alkaline phosphate activity and matrix mineralization was only observed in the LPS plus TNF-α preconditioned MSCs. Mechanistic studies showed that increased prostaglandin E2 (PGE2) production was associated with enhanced Arginase 1 expression. Selective cyclooxygenase-2 inhibition by Celecoxib decreased PGE2 production and Arginase 1 expression in cocultured macrophages.
Conclusions: The novel preconditioned MSCs have increased immunomodulation and bone regeneration potential and could be applied to the treatment of inflammatory bone disorders including periprosthetic osteolysis, fracture healing/nonunions, and osteonecrosis.
Keywords: Immunomodulation; Macrophage polarization; Mesenchymal stem cells; Osteogenesis; Prostaglandin E2.
Conflict of interest statement
Ethics approval
Stanford’s Administrative Panel on Laboratory Animal Care (APLAC) approved the animal cell isolation protocol (APLAC 17566).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization.Stem Cell Res Ther. 2018 Oct 25;9(1):286. doi: 10.1186/s13287-018-1039-2. Stem Cell Res Ther. 2018. PMID: 30359316 Free PMC article.
-
[Adipose-derived stem cells promote the polarization from M1 macrophages to M2 macrophages].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016 Mar;32(3):332-8. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016. PMID: 26927552 Chinese.
-
Trained murine mesenchymal stem cells have anti-inflammatory effect on macrophages, but defective regulation on T-cell proliferation.FASEB J. 2019 Mar;33(3):4203-4211. doi: 10.1096/fj.201801845R. Epub 2018 Dec 6. FASEB J. 2019. PMID: 30521384 Free PMC article.
-
Immunomodulatory Effects of MSCs in Bone Healing.Int J Mol Sci. 2019 Nov 2;20(21):5467. doi: 10.3390/ijms20215467. Int J Mol Sci. 2019. PMID: 31684035 Free PMC article. Review.
-
The Multifunction Role of Tumor-Associated Mesenchymal Stem Cells and Their Interaction with Immune Cells in Breast Cancer.Immunol Invest. 2023 Nov;52(7):856-878. doi: 10.1080/08820139.2023.2249025. Epub 2023 Aug 24. Immunol Invest. 2023. PMID: 37615117 Review.
Cited by
-
Different Effects of Intramedullary Injection of Mesenchymal Stem Cells During the Acute vs. Chronic Inflammatory Phase on Bone Healing in the Murine Continuous Polyethylene Particle Infusion Model.Front Cell Dev Biol. 2021 Mar 19;9:631063. doi: 10.3389/fcell.2021.631063. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33816480 Free PMC article.
-
Preconditioning of Rat Bone Marrow-Derived Mesenchymal Stromal Cells with Toll-Like Receptor Agonists.Stem Cells Int. 2019 Aug 19;2019:7692973. doi: 10.1155/2019/7692973. eCollection 2019. Stem Cells Int. 2019. PMID: 31531025 Free PMC article.
-
Different priming strategies improve distinct therapeutic capabilities of mesenchymal stromal/stem cells: Potential implications for their clinical use.World J Stem Cells. 2023 May 26;15(5):400-420. doi: 10.4252/wjsc.v15.i5.400. World J Stem Cells. 2023. PMID: 37342218 Free PMC article. Review.
-
Modulation of the Inflammatory Response and Bone Healing.Front Endocrinol (Lausanne). 2020 Jun 11;11:386. doi: 10.3389/fendo.2020.00386. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32655495 Free PMC article. Review.
-
SrFe12O19-doped nano-layered double hydroxide/chitosan layered scaffolds with a nacre-mimetic architecture guide in situ bone ingrowth and regulate bone homeostasis.Mater Today Bio. 2022 Jul 19;16:100362. doi: 10.1016/j.mtbio.2022.100362. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 35937572 Free PMC article.
References
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

