Tetraspanin CD63 Bridges Autophagic and Endosomal Processes To Regulate Exosomal Secretion and Intracellular Signaling of Epstein-Barr Virus LMP1
- PMID: 29212935
- PMCID: PMC5809724
- DOI: 10.1128/JVI.01969-17
Tetraspanin CD63 Bridges Autophagic and Endosomal Processes To Regulate Exosomal Secretion and Intracellular Signaling of Epstein-Barr Virus LMP1
Abstract
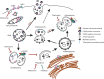
The tetraspanin protein CD63 has been recently described as a key factor in extracellular vesicle (EV) production and endosomal cargo sorting. In the context of Epstein-Barr virus (EBV) infection, CD63 is required for the efficient packaging of the major viral oncoprotein latent membrane protein 1 (LMP1) into exosomes and other EV populations and acts as a negative regulator of LMP1 intracellular signaling. Accumulating evidence has also pointed to intersections of the endosomal and autophagy pathways in maintaining cellular secretory processes and as sites for viral assembly and replication. Indeed, LMP1 can activate the mammalian target of rapamycin (mTOR) pathway to suppress host cell autophagy and facilitate cell growth and proliferation. Despite the growing recognition of cross talk between endosomes and autophagosomes and its relevance to viral infection, little is understood about the molecular mechanisms governing endosomal and autophagy convergence. Here, we demonstrate that CD63-dependent vesicle protein secretion directly opposes intracellular signaling activation downstream of LMP1, including mTOR-associated proteins. Conversely, disruption of normal autolysosomal processes increases LMP1 secretion and dampens signal transduction by the viral protein. Increases in mTOR activation following CD63 knockout are coincident with the development of serum-dependent autophagic vacuoles that are acidified in the presence of high LMP1 levels. Altogether, these findings suggest a key role of CD63 in regulating the interactions between endosomal and autophagy processes and limiting cellular signaling activity in both noninfected and virally infected cells.IMPORTANCE The close connection between extracellular vesicles and viruses is becoming rapidly and more widely appreciated. EBV, a human gamma herpesvirus that contributes to the progression of a multitude of lymphomas and carcinomas in immunocompromised or genetically susceptible populations, packages its major oncoprotein, LMP1, into vesicles for secretion. We have recently described a role of the host cell protein CD63 in regulating intracellular signaling of the viral oncoprotein by shuttling LMP1 into exosomes. Here, we provide strong evidence of the utility of CD63-dependent EVs in regulating global intracellular signaling, including mTOR activation by LMP1. We also demonstrate a key role of CD63 in coordinating endosomal and autophagic processes to regulate LMP1 levels within the cell. Overall, this study offers new insights into the complex intersection of cellular secretory and degradative mechanisms and the implications of these processes in viral replication.
Keywords: Epstein-Barr virus; amphisomes; exosomes; extracellular vesicles; herpesvirus; herpesviruses; multivesicular bodies.
Copyright © 2018 American Society for Microbiology.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

