Annexin A1 is a novel target gene of gonadotropin-releasing hormone in LβT2 gonadotrope cells
- PMID: 29213013
- PMCID: PMC5797869
- DOI: 10.1292/jvms.17-0569
Annexin A1 is a novel target gene of gonadotropin-releasing hormone in LβT2 gonadotrope cells
Abstract
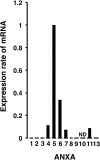
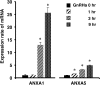
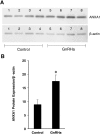
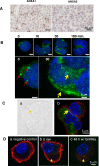
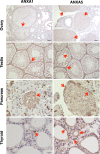
Gonadotropin-releasing hormone (GnRH) regulates gonadotropin secretion. We previously demonstrated that the expression of annexin A5 (ANXA5) is stimulated by GnRH in gonadotropes and has a significant role in gonadotropin secretion. It is therefore of interest to know whether other members of the ANXA family, which consists of twelve structurally related members, are also regulated by GnRH. Therefore, the expression of all annexins was examined in LβT2 gonadotrope cells. ANXA4, A5, A6, A7 and A11 were detected in LβT2 cells. The expression of ANXA5 and A1 mRNA was stimulated by a GnRH agonist. An increase in ANXA1 protein by this agonist was demonstrated by western blotting. Immunohistochemistry showed that ANXA1 was present in the nucleus and to a lesser extent in the cytoplasm of some rat pituitary cells. The GnRH agonist induced translocation of ANXA1 to the periphery of LβT2 cells. The presence of ANXA1 in gonadotropes and its increase upon GnRH agonist treatment were confirmed in a primary pituitary cell culture. ANXA1 expression was also demonstrated in the ovary, the testis, the thyroid gland and the pancreas in a different manner to that of ANXA5. These data suggest that ANXA1 is a novel GnRH target gene in gonadotropes. ANXA1 also may be a target of local GnRH in peripheral tissues and may have a different role than that of ANXA5.
Keywords: GnRH; annexin A1; annexin A5; cell biology; gonadotrope.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

