In vivo evidence for homo- and heterodimeric interactions of Arabidopsis thaliana dehydrins AtCOR47, AtERD10, and AtRAB18
- PMID: 29213048
- PMCID: PMC5719087
- DOI: 10.1038/s41598-017-15986-2
In vivo evidence for homo- and heterodimeric interactions of Arabidopsis thaliana dehydrins AtCOR47, AtERD10, and AtRAB18
Abstract
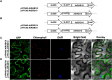
Dehydrins (DHNs) are intrinsically disordered proteins that play central roles in plant abiotic stress responses; however, how they work remains unclear. Herein, we report the in planta subcellular localization of Arabidopsis thaliana DHNs AtCOR47, AtERD10, and AtRAB18 through GFP translational fusions. To explore the dimerization ability of the Arabidopsis acidic DHNs AtCOR47 and AtERD10, we conducted an in planta DHN binding assay using the Bimolecular Fluorescence Complementation (BiFC) technique. Our analyses revealed homodimeric interactions for AtCOR47 and AtERD10; interestingly, heterodimeric associations also occurred with these DHNs, and these interactions were observed in the cytosol of tobacco cells. Furthermore, we evaluated whether Arabidopsis basic DHNs, such as AtRAB18, could also interact with itself and/or with AtCOR47 and AtERD10 in the BiFC system. Our data revealed homodimeric RAB18 complexes in the nucleus and cytosol, while heterodimeric associations between AtRAB18 and acidic DHNs occurred only in the cytosol. Finally, we demonstrated the presence of heterodimeric complexes among Arabidopsis AtCOR47, AtERD10, and AtRAB18 DHNs with their acidic ortholog the OpsDHN1 from Opuntia streptacantha; these heterodimeric interactions showed different subcellular distributions. Our results guide DHN research toward a new scenario where DHN/DHN oligomerization could be explored as a part of their molecular mechanism.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures










References
-
- Dhanaraj AL, Slovin JP, Rowland LJ. Analysis of gene expression associated with cold acclimation in blueberry floral buds using expressed sequence tags. Plant Sci. 2004;166:863–872. doi: 10.1016/j.plantsci.2003.11.013. - DOI
-
- Arumingtyas EL, Savitri ES, Purwoningrahayu RD. Protein profiles and dehydrin accumulation in some soybean varieties (Glycine max L. Merr) in drought stress conditions. Am J Plant Sci. 2013;4:134–141. doi: 10.4236/ajps.2013.41018. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

