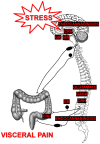
Stress-Induced Chronic Visceral Pain of Gastrointestinal Origin
- PMID: 29213232
- PMCID: PMC5702626
- DOI: 10.3389/fnsys.2017.00086
Stress-Induced Chronic Visceral Pain of Gastrointestinal Origin
Abstract
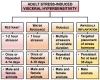
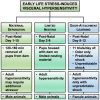
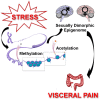
Visceral pain is generally poorly localized and characterized by hypersensitivity to a stimulus such as organ distension. In concert with chronic visceral pain, there is a high comorbidity with stress-related psychiatric disorders including anxiety and depression. The mechanisms linking visceral pain with these overlapping comorbidities remain to be elucidated. Evidence suggests that long term stress facilitates pain perception and sensitizes pain pathways, leading to a feed-forward cycle promoting chronic visceral pain disorders such as irritable bowel syndrome (IBS). Early life stress (ELS) is a risk-factor for the development of IBS, however the mechanisms responsible for the persistent effects of ELS on visceral perception in adulthood remain incompletely understood. In rodent models, stress in adult animals induced by restraint and water avoidance has been employed to investigate the mechanisms of stress-induce pain. ELS models such as maternal separation, limited nesting, or odor-shock conditioning, which attempt to model early childhood experiences such as neglect, poverty, or an abusive caregiver, can produce chronic, sexually dimorphic increases in visceral sensitivity in adulthood. Chronic visceral pain is a classic example of gene × environment interaction which results from maladaptive changes in neuronal circuitry leading to neuroplasticity and aberrant neuronal activity-induced signaling. One potential mechanism underlying the persistent effects of stress on visceral sensitivity could be epigenetic modulation of gene expression. While there are relatively few studies examining epigenetically mediated mechanisms involved in visceral nociception, stress-induced visceral pain has been linked to alterations in DNA methylation and histone acetylation patterns within the brain, leading to increased expression of pro-nociceptive neurotransmitters. This review will discuss the potential neuronal pathways and mechanisms responsible for stress-induced exacerbation of chronic visceral pain. Additionally, we will review the importance of specific experimental models of adult stress and ELS in enhancing our understanding of the basic molecular mechanisms of pain processing.
Keywords: animal model; brain; colon; early life; gastrointestinal tract; irritable bowel syndrome; pain; stress.
Figures
Similar articles
-
Mechanisms of Stress-induced Visceral Pain.J Neurogastroenterol Motil. 2018 Jan 30;24(1):7-18. doi: 10.5056/jnm17137. J Neurogastroenterol Motil. 2018. PMID: 29291604 Free PMC article. Review.
-
Epigenetic mechanisms underlying stress-induced visceral pain: Resilience versus vulnerability in a two-hit model of early life stress and chronic adult stress.Neurogastroenterol Motil. 2023 May;35(5):e14558. doi: 10.1111/nmo.14558. Epub 2023 Mar 9. Neurogastroenterol Motil. 2023. PMID: 36893055
-
The Pharmacology of Visceral Pain.Adv Pharmacol. 2016;75:273-301. doi: 10.1016/bs.apha.2015.11.002. Epub 2016 Jan 6. Adv Pharmacol. 2016. PMID: 26920016 Review.
-
Contribution of Amygdala Histone Acetylation in Early Life Stress-Induced Visceral Hypersensitivity and Emotional Comorbidity.Front Neurosci. 2022 May 6;16:843396. doi: 10.3389/fnins.2022.843396. eCollection 2022. Front Neurosci. 2022. PMID: 35600618 Free PMC article.
-
Sex-related differences in pain behaviors following three early life stress paradigms.Biol Sex Differ. 2016 Jun 10;7:29. doi: 10.1186/s13293-016-0082-x. eCollection 2016. Biol Sex Differ. 2016. PMID: 27293543 Free PMC article.
Cited by
-
Possible implications of animal models for the assessment of visceral pain.Animal Model Exp Med. 2020 Aug 10;3(3):215-228. doi: 10.1002/ame2.12130. eCollection 2020 Sep. Animal Model Exp Med. 2020. PMID: 33024943 Free PMC article. Review.
-
Importance of Non-pharmacological Approaches for Treating Irritable Bowel Syndrome: Mechanisms and Clinical Relevance.Front Pain Res (Lausanne). 2021 Jan 21;1:609292. doi: 10.3389/fpain.2020.609292. eCollection 2020. Front Pain Res (Lausanne). 2021. PMID: 35295688 Free PMC article. Review.
-
Upregulation of Netrin-1 in the hippocampus mediates the formation of visceral hypersensitivity induced by maternal separation.Front Mol Neurosci. 2022 Jul 28;15:908911. doi: 10.3389/fnmol.2022.908911. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35966013 Free PMC article.
-
Vagal gut-brain signaling mediates amygdaloid plasticity, affect, and pain in a functional dyspepsia model.JCI Insight. 2021 Mar 22;6(6):e144046. doi: 10.1172/jci.insight.144046. JCI Insight. 2021. PMID: 33591956 Free PMC article.
-
The effect of self-management online modules plus nurse-led support on pain and quality of life among young adults with irritable bowel syndrome: A randomized controlled trial.Int J Nurs Stud. 2022 Aug;132:104278. doi: 10.1016/j.ijnurstu.2022.104278. Epub 2022 Apr 30. Int J Nurs Stud. 2022. PMID: 35640500 Free PMC article. Clinical Trial.
References
-
- Ackerman A. L., Jellison F. C., Lee U. J., Bradesi S., Rodriguez L. V. (2016). The Glt1 glutamate receptor mediates the establishment and perpetuation of chronic visceral pain in an animal model of stress-induced bladder hyperalgesia. Am. J. Physiol. Renal Physiol. 310, F628–F636. 10.1152/ajprenal.00297.2015 - DOI - PMC - PubMed
-
- Agostini S., Goubern M., Tondereau V., Salvador-Cartier C., Bezirard V., Leveque M., et al. . (2012). A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol. Motil. 24, 376–e172. 10.1111/j.1365-2982.2011.01865.x - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

