The NIMO Scandinavian Study: A Prospective Observational Study of Iron Isomaltoside Treatment in Patients with Iron Deficiency
- PMID: 29213281
- PMCID: PMC5682076
- DOI: 10.1155/2017/4585164
The NIMO Scandinavian Study: A Prospective Observational Study of Iron Isomaltoside Treatment in Patients with Iron Deficiency
Abstract
Background: Intravenous iron allows for efficient and well-tolerated treatment in iron deficiency and is routinely used in diseases of the gastrointestinal tract.
Objective: The aims of this study were to determine the probability of relapse of iron deficiency over time and to investigate treatment routine, effectiveness, and safety of iron isomaltoside.
Methods: A total of 282 patients treated with iron isomaltoside were observed for two treatments or a minimum of one year.
Results: Out of 282 patients, 82 had Crohn's disease and 67 had ulcerative colitis. Another 133 patients had chronic blood loss, malabsorption, or malignancy. Patients who received an iron isomaltoside dose above 1000 mg had a 65% lower probability of needing retreatment compared with those given 1000 mg. A clinically significant treatment response was shown, but in 71/191 (37%) of patients, anaemia was not corrected. The mean dose given was 1100 mg, lower than the calculated total iron need of 1481 mg. Adverse drug reactions were reported in 4% of patients.
Conclusion: Iron isomaltoside is effective with a good safety profile, and high doses reduce the need for retreatment over time. Several patients were anaemic after treatment, indicating that doses were inadequate for full iron correction. This trial is registered with NCT01900197.
Figures
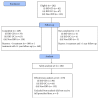
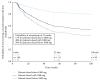

References
-
- Bager P., Befrits R., Wikman O., et al. High burden of iron deficiency and different types of anemia in inflammatory bowel disease outpatients in Scandinavia: a longitudinal 2-year follow-up study. Scandinavian Journal of Gastroenterology. 2013;48(11):1286–1293. doi: 10.3109/00365521.2013.838605. - DOI - PubMed
-
- Ott C., Liebold A., Takses A., Strauch U. G., Obermeier F. High prevalence but insufficient treatment of iron-deficiency anemia in patients with inflammatory bowel disease: results of a population-based cohort. Gastroenterology Research and Practice. 2012;2012:7. doi: 10.1155/2012/595970.595970 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

