Endometritis and In Vitro PGE2 Challenge Modify Properties of Cattle Endometrial Mesenchymal Stem Cells and Their Transcriptomic Profile
- PMID: 29213289
- PMCID: PMC5682089
- DOI: 10.1155/2017/4297639
Endometritis and In Vitro PGE2 Challenge Modify Properties of Cattle Endometrial Mesenchymal Stem Cells and Their Transcriptomic Profile
Abstract
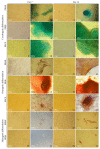
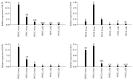
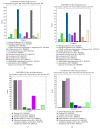
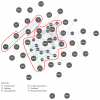
Mesenchymal stem cells (MSCs) were isolated and characterized from postpartum bovine endometrium of animals with subclinical (n = 5) and clinical endometritis (n = 3) and healthy puerperal females (n = 5). Cells isolated displayed mean morphological features of MSCs and underwent osteogenic, chondrogenic, and adipogenic differentiation after induction (healthy and subclinical). Cells from cows with clinical endometritis did not undergo adipogenic differentiation. All cells expressed mRNAs for selected MSC markers. Endometrial MSCs were challenged in vitro with PGE2 at concentrations of 0, 1, 3, and 10 μM, and their global transcriptomic profile was studied. Overall, 1127 genes were differentially expressed between unchallenged cells and cells treated with PGE2 at all concentrations (763 up- and 364 downregulated, fold change > 2, and P < 0.05). The pathways affected the most by the PGE2 challenge were immune response, angiogenesis, and cell proliferation. In conclusion, we demonstrated that healthy puerperal bovine endometrium contains MSCs and that endometritis modifies and limits some functional characteristics of these cells, such as their ability to proceed to adipogenic differentiation. Also, PGE2, an inflammatory mediator of endometritis, modifies the transcriptomic profile of endometrial MSCs. A similar situation may occur during inflammation associated with endometritis, therefore affecting the main properties of endometrial MSCs.
Figures



References
-
- Okano A., Tomizuka T. Post partum uterine involution in the cow. Theriogenology. 1996;27(2):369–376. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

