Green route to synthesis of valuable chemical 6-hydroxynicotine from nicotine in tobacco wastes using genetically engineered Agrobacterium tumefaciens S33
- PMID: 29213327
- PMCID: PMC5713474
- DOI: 10.1186/s13068-017-0976-9
Green route to synthesis of valuable chemical 6-hydroxynicotine from nicotine in tobacco wastes using genetically engineered Agrobacterium tumefaciens S33
Abstract
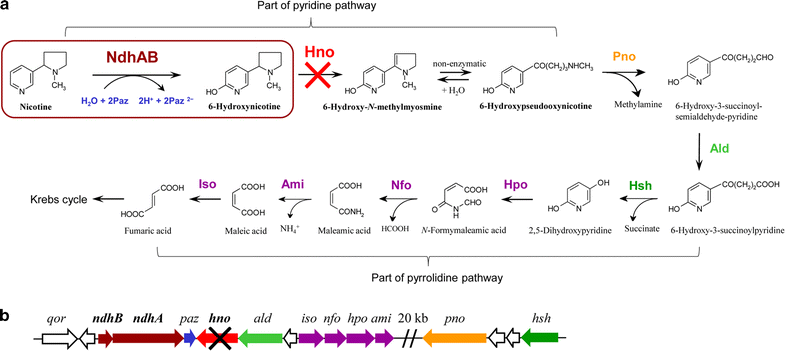
Background: Tobacco is widely planted as an important nonfood economic crop throughout the world, and large amounts of tobacco wastes are generated during the tobacco manufacturing process. Tobacco and its wastes contain high nicotine content. This issue has become a major concern for health and environments due to its toxicity and complex physiological effects. The microbial transformation of nicotine into valuable functionalized pyridine compounds is a promising way to utilize tobacco and its wastes as a potential biomass resource. Agrobacterium tumefaciens S33 is able to degrade nicotine via a novel hybrid of the pyridine and pyrrolidine pathways, in which several intermediates, such as 6-hydroxynicotine, can be used as renewable precursors to synthesize drugs and insecticides. This provides an opportunity to produce valuable chemical 6-hydroxynicotine from nicotine via biocatalysis using strain S33.
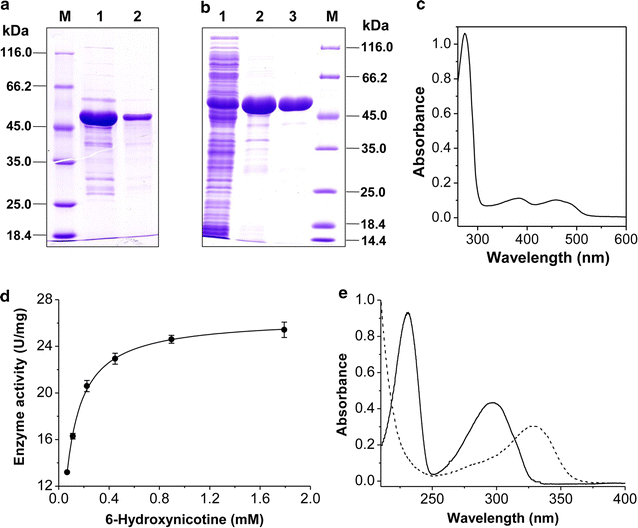
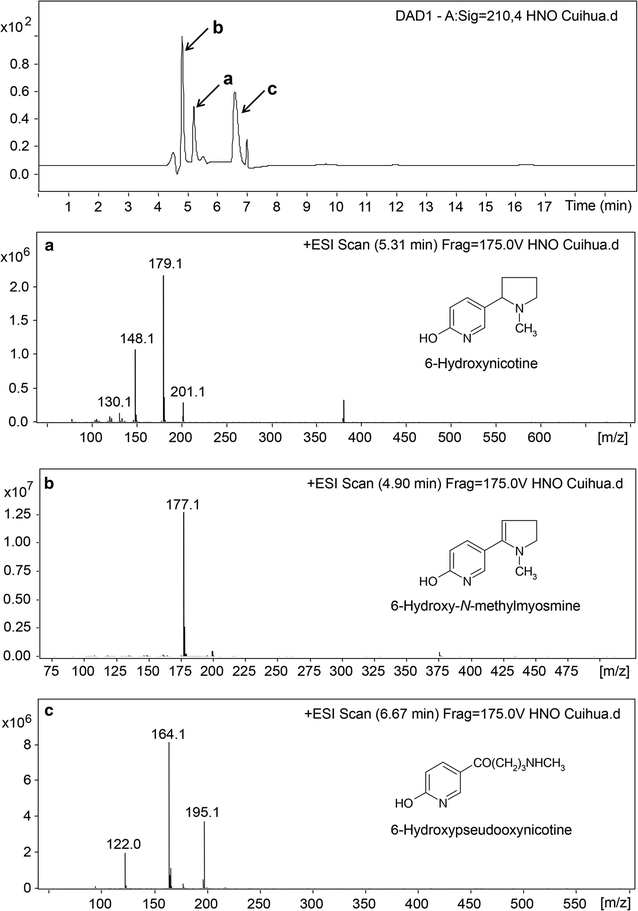
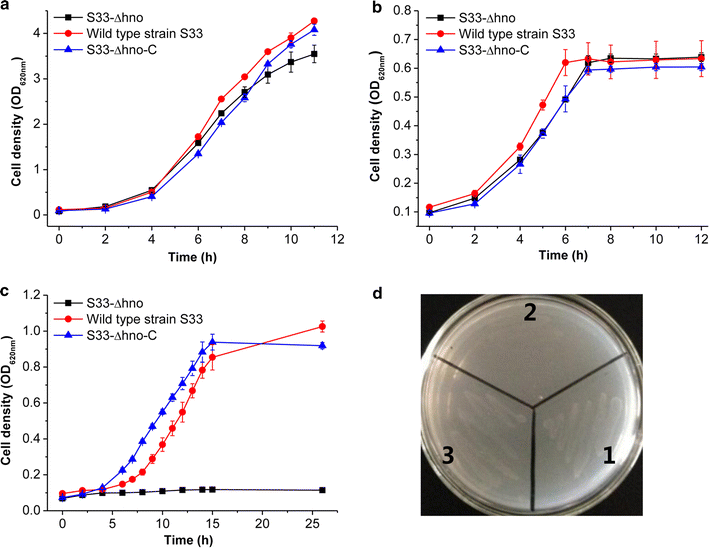
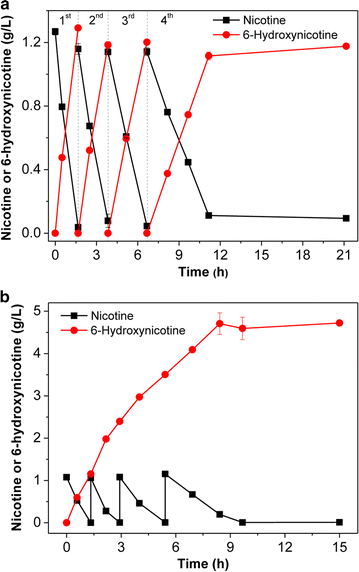
Results: To accumulate the intermediate 6-hydroxynicotine, we firstly identified the key enzyme decomposing 6-hydroxynicotine, named 6-hydroxynicotine oxidase, and then disrupted its encoding gene in A. tumefaciens S33. With the whole cells of the mutant as a biocatalyst, we tested the possibility to produce 6-hydroxynicotine from the nicotine of tobacco and its wastes and optimized the reaction conditions. At 30 °C and pH 7.0, nicotine could be efficiently transformed into 6-hydroxynicotine by the whole cells cultivated with glucose/ammonium/6-hydroxy-3-succinoylpyridine medium. The molar conversion and the specific catalytic rate reached approximately 98% and 1.01 g 6-hydroxynicotine h-1 g-1 dry cells, respectively. The product could be purified easily by dichloromethane extraction with a recovery of 76.8%, and was further confirmed by UV spectroscopy, mass spectroscopy, and NMR analysis.
Conclusions: We successfully developed a novel biocatalytic route to 6-hydroxynicotine from nicotine by blocking the nicotine catabolic pathway via gene disruption, which provides an alternative green strategy to utilize tobacco and its wastes as a biomass resource by converting nicotine into valuable hydroxylated-pyridine compounds.
Keywords: 6-Hydroxynicotine; 6-Hydroxynicotine oxidase; Agrobacterium tumefaciens; Biotransformation; Functionalized pyridine; Nicotine; Tobacco wastes.
Figures



References
-
- Civilini M, Domenis C, Sebastianutto N, de Berfoldi M. Nicotine decontamination of tobacco agro-industrial waste and its degradation by micro-organisms. Waste Manag Res. 1997;15:349–358. doi: 10.1177/0734242X9701500403. - DOI
-
- Food and Agriculture Organization . Projections of tobacco production, consumption and trade to the year 2010. Rome: Food and Agriculture Organization of the United Nations; 2003.
LinkOut - more resources
Full Text Sources
Other Literature Sources

