Cell-based technologies for Huntington's disease
- PMID: 29213471
- PMCID: PMC5619267
- DOI: 10.1590/s1980-5764-2016dn1004006
Cell-based technologies for Huntington's disease
Abstract
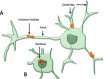
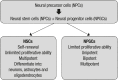
Huntington's disease (HD) is a fatal genetic disorder, which causes the progressive breakdown of neurons in the human brain. HD deteriorates human physical and mental abilities over time and has no cure. Stem cell-based technologies are promising novel treatments, and in HD, they aim to replace lost neurons and/or to prevent neural cell death. Herein we discuss the use of human fetal tissue (hFT), neural stem cells (NSCs) of hFT origin or embryonic stem cells (ESCs) and induced pluripotent stem cells (IPSCs), in clinical and pre-clinical studies. The in vivo use of mesenchymal stem cells (MSCs), which are derived from non-neural tissues, will also be discussed. All these studies prove the potential of stem cells for transplantation therapy in HD, demonstrating cell grafting and the ability to differentiate into mature neurons, resulting in behavioral improvements. We claim that there are still many problems to overcome before these technologies become available for HD patient treatment, such as: a) safety regarding the use of NSCs and pluripotent stem cells, which are potentially teratogenic;b) safety regarding the transplantation procedure itself, which represents a risk and needs to be better studied; and finallyc) technical and ethical issues regarding cells of fetal and embryonic origin.
A doença de Huntington (DH) é uma desordem genética que provoca a destruição progressiva dos neurônios no cérebro humano. A DH deteriora progressivamente as habilidades físicas e mentais humanas, e é incurável. Tecnologias terapêuticas baseadas em células representam novas alternativas para diversas doenças neurodegenerativas, pois visam substituir neurônios e/ou prevenir a morte neuronal. Nesta revisão discutirmos o uso de tecido fetal humano, células tronco neurais (CTN) de origem fetal ou de células tronco embrionárias ou células tronco pluripotentes induzidas, em estudos pré-clínicos e clínicos. Além disso, o uso terapêutico de células derivadas de tecidos não-neurais, como células tronco mesenquimais, também será discutido. Todos estes estudos provam o potencial do transplante celular na DH, demonstrando a sua habilidade em enxertar no encéfalo e diferenciar em neurônios in vivo, resultando em melhorias comportamentais e motoras em modelos animais da DH. Nós também discutimos que há muitos problemas a serem resolvidos quanto à terapia celular na DH, tais como:a) questões associadas à segurança do uso de CTNs, as quais são consideradas potencialmente teratogênicas;b) segurança do procedimento de transplante intracerebral, que representa um risco ao paciente;c) e, finalmente, questões técnicas e éticas associadas ao uso de células de origem fetal e embrionária.
Keywords: Huntington's disease; cell therapy; safety issues; stem cells.
Conflict of interest statement
Disclosure: The authors report no conflicts of interest.
Figures

References
-
- Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57(5):369–384. - PubMed
-
- The Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72(6):971–983. - PubMed
-
- Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. - PubMed
-
- Hayden MR. Huntington's chorea. London: Springer; 1981.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

