Morphological bactericidal fast-acting effects of peracetic acid, a high-level disinfectant, against Staphylococcus aureus and Pseudomonas aeruginosa biofilms in tubing
- PMID: 29214017
- PMCID: PMC5709933
- DOI: 10.1186/s13756-017-0281-1
Morphological bactericidal fast-acting effects of peracetic acid, a high-level disinfectant, against Staphylococcus aureus and Pseudomonas aeruginosa biofilms in tubing
Abstract
Background: The bactericidal effect of disinfectants against biofilms is essential to reduce potential endoscopy-related infections caused by contamination. Here, we investigated the bactericidal effect of a high-level disinfectant, peracetic acid (PAA), against Staphylococcus aureus and Pseudomonas aeruginosa biofilm models in vitro.
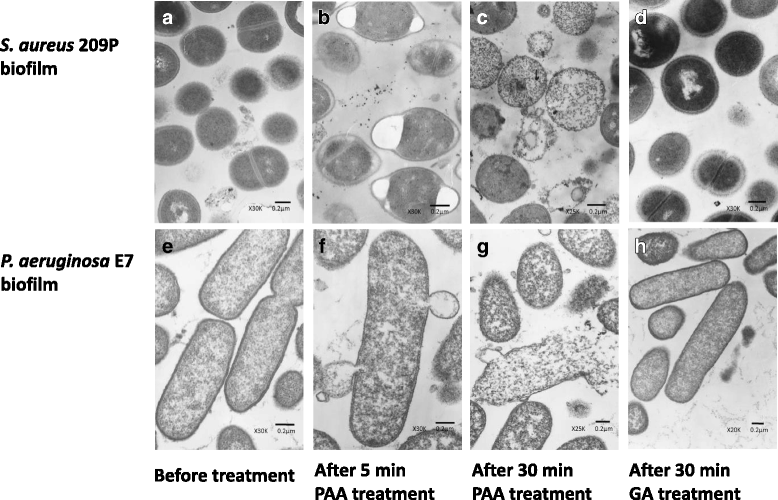
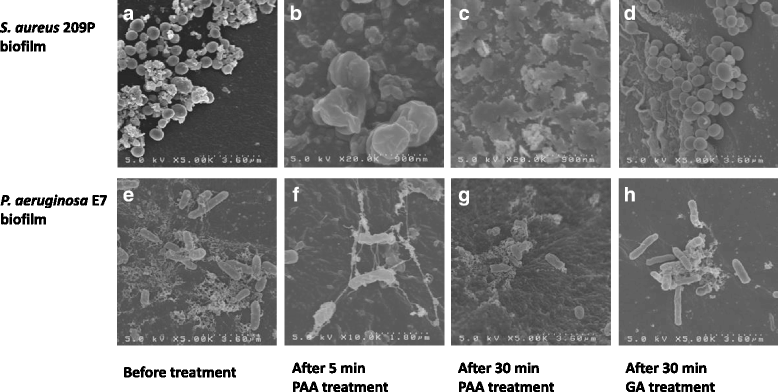
Methods: S. aureus and P. aeruginosa biofilms were cultured at 35 °C for 7 days with catheter tubes. The following high-level disinfectants (HLDs) were tested: 0.3% PAA, 0.55% ortho-phthalaldehyde (OPA), and 2.0% alkaline-buffered glutaraldehyde (GA). Biofilms were exposed to these agents for 1-60 min and observed after 5 min and 30 min by transmission and scanning electron microscopy. A Student's t test was performed to compare the exposure time required for bactericidal effectiveness of the disinfectants.
Results: PAA and GA were active within 1 min and 5 min, respectively, against S. aureus and P. aeruginosa biofilms. OPA took longer than 10 min and 30 min to act against S. aureus and P. aeruginosa biofilms, respectively (p < 0.01). Treatment with PAA elicited changes in cell shape after 5 min and structural damage after 30 min.
Conclusions: Amongst the HLDs investigated, PAA elicited the most rapid bactericidal effects against both biofilms. Additionally, treatment with PAA induced morphological alterations in the in vitro biofilm models, suggesting that PAA exerts fast-acting bactericidal effects against biofilms associated with endoscopy-related infections. These findings indicate that the exposure time for bactericidal effectiveness of HLDs for endoscope reprocessing in healthcare settings should be reconsidered.
Keywords: Bactericidal effectiveness; Electron microscopy; Exposure time; Peracetic acid; Pseudomonas aeruginosa biofilm; Staphylococcus aureus biofilm.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

