The design of reversible hydrogels to capture extracellular matrix dynamics
- PMID: 29214058
- PMCID: PMC5714327
- DOI: 10.1038/natrevmats.2015.12
The design of reversible hydrogels to capture extracellular matrix dynamics
Abstract
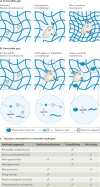
The extracellular matrix (ECM) is a dynamic environment that constantly provides physical and chemical cues to embedded cells. Much progress has been made in engineering hydrogels that can mimic the ECM, but hydrogel properties are, in general, static. To recapitulate the dynamic nature of the ECM, many reversible chemistries have been incorporated into hydrogels to regulate cell spreading, biochemical ligand presentation and matrix mechanics. For example, emerging trends include the use of molecular photoswitches or biomolecule hybridization to control polymer chain conformation, thereby enabling the modulation of the hydrogel between two states on demand. In addition, many non-covalent, dynamic chemical bonds have found increasing use as hydrogel crosslinkers or tethers for cell signalling molecules. These reversible chemistries will provide greater temporal control of adhered cell behaviour, and they allow for more advanced in vitro models and tissue-engineering scaffolds to direct cell fate.
Conflict of interest statement
Competing interests statement The authors declare no competing interests.
Figures
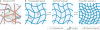

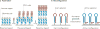



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
