Pseudomonas aeruginosa zinc uptake in chelating environment is primarily mediated by the metallophore pseudopaline
- PMID: 29214991
- PMCID: PMC5719457
- DOI: 10.1038/s41598-017-16765-9
Pseudomonas aeruginosa zinc uptake in chelating environment is primarily mediated by the metallophore pseudopaline
Abstract
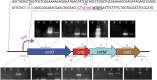
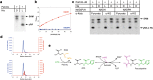
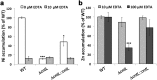
Metal uptake is vital for all living organisms. In metal scarce conditions a common bacterial strategy consists in the biosynthesis of metallophores, their export in the extracellular medium and the recovery of a metal-metallophore complex through dedicated membrane transporters. Staphylopine is a recently described metallophore distantly related to plant nicotianamine that contributes to the broad-spectrum metal uptake capabilities of Staphylococcus aureus. Here we characterize a four-gene operon (PA4837-PA4834) in Pseudomonas aeruginosa involved in the biosynthesis and trafficking of a staphylopine-like metallophore named pseudopaline. Pseudopaline differs from staphylopine with regard to the stereochemistry of its histidine moiety associated with an alpha ketoglutarate moiety instead of pyruvate. In vivo, the pseudopaline operon is regulated by zinc through the Zur repressor. The pseudopaline system is involved in nickel uptake in poor media, and, most importantly, in zinc uptake in metal scarce conditions mimicking a chelating environment, thus reconciling the regulation of the cnt operon by zinc with its function as the main zinc importer under these metal scarce conditions.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Budzikiewicz, A. G. Siderophores from bacteria and from fungi. In Iron uptake and homeostasis in microorganisms 1–16 (Cornelis, P. and Andrews, S. C.), (2010).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

