Differential Raman backscattering cross sections of black carbon nanoparticles
- PMID: 29215038
- PMCID: PMC5719417
- DOI: 10.1038/s41598-017-17300-6
Differential Raman backscattering cross sections of black carbon nanoparticles
Abstract
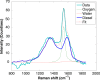
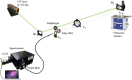
We report the measurements of the differential Raman backscattering cross sections for several carbonaceous ultrafine particles of environmental relevances. These were obtained by dispersing the target particles in liquid water which was used as the internal standard reference. The optical collection was performed in a configuration to ensure a detection as close as possible to the backward direction. These are the first cross sections on black carbon-type particles although Raman spectroscopy is widely used in Carbon science. The high values of the cross sections, few 10-28 cm2.sr-1.atom-1, reflect resonance effects that take advantages of the disordered polyaromatic structures. Because they were measured in conditions intended to mimic the aerosol phase, these measurements provide a crucial step to move toward quantitative Raman spectroscopy and enable development of dedicated teledetection of black carbon in the atmosphere and in combustion chambers.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Ehrenfreund P, Charnley S. Organic molecules in the interstellar medium, comets, and meteorites: A voyage from dark clouds to the early earth. Ann. Rev. Astrophys. Astron. 2000;38:427. doi: 10.1146/annurev.astro.38.1.427. - DOI
-
- Delhaes, P. Carbon-based Solids and Materials (John Wiley& Sons, Ltd, 2011).
-
- Bond TC, et al. Bounding the role of black carbon in the climate system: A scientific assessment. Journal of geophysical research. 2013;118:5380–5552.
-
- Buseck PR, Adachi K, Gelencsér A, Tompa É, Pósfai M. Ns-Soot: A Material-Based Term for Strongly Light-Absorbing Carbonaceous Particles. Aerosol Science and Technology. 2014;48:777–788. doi: 10.1080/02786826.2014.919374. - DOI
-
- Reid JS, et al. Observing and understanding the Southeast Asian aerosol system by remote sensing: An initial review and analysis for the Seven Southeast Asian Studies (7SEAS) program. Atmospheric Research. 2013;122:403–468. doi: 10.1016/j.atmosres.2012.06.005. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

