Sex-related pharmacokinetic differences and mechanisms of metapristone (RU486 metabolite)
- PMID: 29215040
- PMCID: PMC5719405
- DOI: 10.1038/s41598-017-17225-0
Sex-related pharmacokinetic differences and mechanisms of metapristone (RU486 metabolite)
Abstract
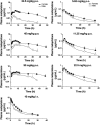
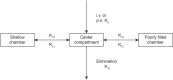
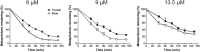
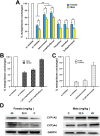
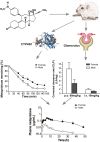
Metapristone is the primary metabolite of the abortifacient mifepristone (RU486), and is being developed as a safe and effective cancer metastatic chemopreventive agent for both sexes. Here, we systematically investigated the sex-related pharmacokinetics of metapristone in both rats and dogs, and explored the related mechanisms of actions. Administration of metapristone to rats and dogs showed that plasma concentrations of metapristone (AUC, C max ) were significantly higher in female dogs and rats than in males. The sex-related differences in pharmacokinetics become more significant after ten consecutive days of oral administration. Female liver microsomes metabolized metapristone significantly slower than the male ones. The results from P450 reaction phenotyping using recombinant cDNA-expressed human CYPs in conjunction with specific CYP inhibitors suggested that CYP1A2 and CYP3A4 are the predominant CYPs involved in the metapristone metabolism, which were further confirmed by the enhanced protein levels of CYP1A2 and CYP3A4 induced by 1-week oral administration of metapristone to rats. The highest tissue concentration of metapristone was found in the liver. The study demonstrates, for the first time, the sex-related pharmacokinetics of metapristone, and reveals that activities of liver microsomal CYP1A2 and CYP3A4 as well as the renal clearance are primarily responsible for the sex-related pharmacokinetics.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
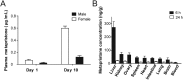
Similar articles
-
Pharmacokinetic differences of mifepristone between sexes in animals.J Pharm Biomed Anal. 2018 May 30;154:108-115. doi: 10.1016/j.jpba.2018.03.008. Epub 2018 Mar 8. J Pharm Biomed Anal. 2018. PMID: 29544105
-
A novel UPLC/MS/MS method for rapid determination of metapristone in rat plasma, a new cancer metastasis chemopreventive agent derived from mifepristone (RU486).J Pharm Biomed Anal. 2014 Jul;95:158-63. doi: 10.1016/j.jpba.2014.02.026. Epub 2014 Mar 12. J Pharm Biomed Anal. 2014. PMID: 24682015
-
In vitro and in vivo efficacy and safety evaluation of metapristone and mifepristone as cancer metastatic chemopreventive agents.Biomed Pharmacother. 2016 Mar;78:291-300. doi: 10.1016/j.biopha.2016.01.017. Epub 2016 Feb 4. Biomed Pharmacother. 2016. PMID: 26898454
-
Antiprogestin pharmacodynamics, pharmacokinetics, and metabolism: implications for their long-term use.J Pharmacokinet Biopharm. 1997 Dec;25(6):647-72. doi: 10.1023/a:1025725716343. J Pharmacokinet Biopharm. 1997. PMID: 9697076 Review.
-
The unique pharmacological characteristics of mifepristone (RU486): from terminating pregnancy to preventing cancer metastasis.Med Res Rev. 2014 Sep;34(5):979-1000. doi: 10.1002/med.21311. Epub 2014 Mar 1. Med Res Rev. 2014. PMID: 24585714 Review.
Cited by
-
Unexpected differences in the pharmacokinetics of N-acetyl-DL-leucine enantiomers after oral dosing and their clinical relevance.PLoS One. 2020 Feb 27;15(2):e0229585. doi: 10.1371/journal.pone.0229585. eCollection 2020. PLoS One. 2020. PMID: 32108176 Free PMC article.
-
Modulation of glucocorticoid receptor in human epileptic endothelial cells impacts drug biotransformation in an in vitro blood-brain barrier model.Epilepsia. 2018 Nov;59(11):2049-2060. doi: 10.1111/epi.14567. Epub 2018 Sep 28. Epilepsia. 2018. PMID: 30264400 Free PMC article.
-
Pharmacokinetics of thalidomide in dogs: can feeding affect it? A preliminary study.J Vet Sci. 2020 Sep;21(5):e60. doi: 10.4142/jvs.2020.21.e60. J Vet Sci. 2020. PMID: 33016014 Free PMC article.
-
Metapristone (RU486-derivative) inhibits endometrial cancer cell progress through regulating miR-492/Klf5/Nrf1 axis.Cancer Cell Int. 2021 Jan 7;21(1):29. doi: 10.1186/s12935-020-01682-1. Cancer Cell Int. 2021. PMID: 33413440 Free PMC article.
-
Pharmacokinetics, efficacy, and safety of cannabidiol in dogs: an update of current knowledge.Front Vet Sci. 2023 Jun 30;10:1204526. doi: 10.3389/fvets.2023.1204526. eCollection 2023. Front Vet Sci. 2023. PMID: 37456953 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

