High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study
- PMID: 29215048
- PMCID: PMC5719364
- DOI: 10.1038/s41598-017-17568-8
High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study
Abstract
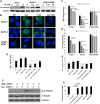
Pancreatic cancer is among the most lethal cancers with poorly tolerated treatments. There is increasing interest in using high-dose intravenous ascorbate (IVC) in treating this disease partially because of its low toxicity. IVC bypasses bioavailability barriers of oral ingestion, provides pharmacological concentrations in tissues, and exhibits selective cytotoxic effects in cancer cells through peroxide formation. Here, we further revealed its anti-pancreatic cancer mechanisms and conducted a phase I/IIa study to investigate pharmacokinetic interaction between IVC and gemcitabine. Pharmacological ascorbate induced cell death in pancreatic cancer cells with diverse mutational backgrounds. Pharmacological ascorbate depleted cellular NAD+ preferentially in cancer cells versus normal cells, leading to depletion of ATP and robustly increased α-tubulin acetylation in cancer cells. While ATP depletion led to cell death, over-acetylated tubulin led to inhibition of motility and mitosis. Collagen was increased, and cancer cell epithelial-mesenchymal transition (EMT) was inhibited, accompanied with inhibition in metastasis. IVC was safe in patients and showed the possibility to prolong patient survival. There was no interference to gemcitabine pharmacokinetics by IVC administration. Taken together, these data revealed a multi-targeting mechanism of pharmacological ascorbate's anti-cancer action, with minimal toxicity, and provided guidance to design larger definitive trials testing efficacy of IVC in treating advanced pancreatic cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- American Cancer Society. Cancer Facts and Figures 2017 (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

