Proxies of quality of life in metastatic colorectal cancer: analyses in the RECOURSE trial
- PMID: 29215098
- PMCID: PMC5708320
- DOI: 10.1136/esmoopen-2017-000261
Proxies of quality of life in metastatic colorectal cancer: analyses in the RECOURSE trial
Abstract
Background: In the pivotal phase III, randomised, double-blind, placebo-controlled RECOURSE study, treatment with trifluridine/tipiracil was well tolerated and associated with prolonged progression-free and overall survival in patients with metastatic colorectal cancer (mCRC). There was no formal analysis of quality of life (QoL) in RECOURSE. The aim of the present analysis was to assess proxies of QoL during the RECOURSE treatment period, in terms of adverse events (AEs) likely to affect QoL and Eastern Cooperative Oncology Group performance status (ECOG PS).
Patients and methods: Enrolled patients had documented, previously treated (≥2 prior chemotherapy lines) mCRC and an ECOG PS of 0 or 1. Patients received best supportive care plus trifluridine/tipiracil 35 mg/m2 twice daily (n=534) or placebo (n=266) in a 28-day cycle. AEs analysed included nausea, vomiting, diarrhoea, dysgeusia and fatigue/asthenia. ECOG PS was determined at baseline, on day 1 of each treatment cycle, at treatment end and 30 days post-treatment discontinuation.
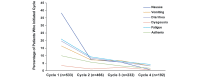
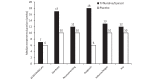
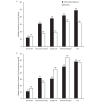
Results: AEs that affect QoL were more frequent in patients treated with trifluridine/tipiracil than placebo. Median treatment duration for patients experiencing at least one of these AEs was longer than that observed for the overall RECOURSE population (trifluridine/tipiracil: 12 vs 7 weeks; placebo: 10 vs 6 weeks). Versus placebo, the duration of most AEs was longer in trifluridine/tipiracil recipients; however, all AEs except nausea and vomiting occupied a lower proportion of the total treatment period. Of the patients who had their PS recorded at discontinuation, PS was maintained in 67% and 63% of trifluridine/tipiracil and placebo recipients, and 84% and 81% of the trifluridine/tipiracil and placebo patients remained at a PS of 0 or 1 at discontinuation.
Conclusions: Analysis of ECOG PS and AEs thought to affect QoL in the RECOURSE patient population suggests that trifluridine/tipiracil treatment does not result in a deterioration of patient QoL versus placebo.
Keywords: advanced colorectal cancer; performance status; quality of life; trifluridine/tipiracil.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment on
-
QTWiST analysis of the RECOURSE trial of trifluridine/tipiracil in metastatic colorectal cancer.ESMO Open. 2017 Nov 23;2(5):e000284. doi: 10.1136/esmoopen-2017-000284. eCollection 2017. ESMO Open. 2017. PMID: 29211817 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

