Host Cell Restriction Factors that Limit Influenza A Infection
- PMID: 29215570
- PMCID: PMC5744151
- DOI: 10.3390/v9120376
Host Cell Restriction Factors that Limit Influenza A Infection
Abstract
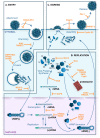
Viral infection of different cell types induces a unique spectrum of host defence genes, including interferon-stimulated genes (ISGs) and genes encoding other proteins with antiviral potential. Although hundreds of ISGs have been described, the vast majority have not been functionally characterised. Cellular proteins with putative antiviral activity (hereafter referred to as "restriction factors") can target various steps in the virus life-cycle. In the context of influenza virus infection, restriction factors have been described that target virus entry, genomic replication, translation and virus release. Genome wide analyses, in combination with ectopic overexpression and/or gene silencing studies, have accelerated the identification of restriction factors that are active against influenza and other viruses, as well as providing important insights regarding mechanisms of antiviral activity. Herein, we review current knowledge regarding restriction factors that mediate anti-influenza virus activity and consider the viral countermeasures that are known to limit their impact. Moreover, we consider the strengths and limitations of experimental approaches to study restriction factors, discrepancies between in vitro and in vivo studies, and the potential to exploit restriction factors to limit disease caused by influenza and other respiratory viruses.
Keywords: influenza; innate; interferon-stimulated gene; replication; restriction factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bright R.A., Medina M.J., Xu X., Perez-Oronoz G., Wallis T.R., Davis X.M., Povinelli L., Cox N.J., Klimov A.I. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: A cause for concern. Lancet. 2005;366:1175–1181. doi: 10.1016/S0140-6736(05)67338-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

