RSV Infection in Human Macrophages Promotes CXCL10/IP-10 Expression during Bacterial Co-Infection
- PMID: 29215596
- PMCID: PMC5751256
- DOI: 10.3390/ijms18122654
RSV Infection in Human Macrophages Promotes CXCL10/IP-10 Expression during Bacterial Co-Infection
Abstract
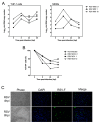
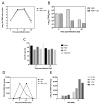
Respiratory syncytial virus (RSV), a major etiologic agent of acute lower respiratory infection constitutes the most important cause of death in young children worldwide. Viral/bacterial mixed infections are related to severity of respiratory inflammatory diseases, but the underlying mechanisms remain poorly understood. We have previously investigated the intracellular mechanisms that mediate the immune response in the context of influenza virus/Streptococcus pneumoniae (Sp) co-infection using a model of human monocyte-derived macrophages (MDMs). Here, we set up and characterized a similar model of MDMs to investigate different scenarios of RSV infection and co-infection with Sp. Our results suggest that Sp contributes to a faster and possibly higher level of CXCL10/IP-10 expression induced by RSV infection in human MDMs.
Keywords: Streptococcus pneumoniae (Sp); acute lower respiratory infection; co-infection; macrophages; respiratory syncytial virus (RSV).
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

