Update: Influenza Activity - United States, October 1-November 25, 2017
- PMID: 29216030
- PMCID: PMC5757637
- DOI: 10.15585/mmwr.mm6648a2
Update: Influenza Activity - United States, October 1-November 25, 2017
Abstract
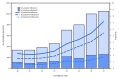
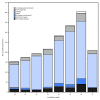
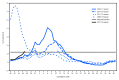
Influenza activity in the United States was low during October 2017, but has been increasing since the beginning of November. Influenza A viruses have been most commonly identified, with influenza A(H3N2) viruses predominating. Several influenza activity indicators were higher than is typically seen for this time of year. The majority of influenza viruses characterized during this period were genetically or antigenically similar to the 2017-18 Northern Hemisphere cell-grown vaccine reference viruses. These data indicate that currently circulating viruses have not undergone significant antigenic drift; however, circulating A(H3N2) viruses are antigenically less similar to egg-grown A(H3N2) viruses used for producing the majority of influenza vaccines in the United States. It is difficult to predict which influenza viruses will predominate in the 2017-18 influenza season; however, in recent past seasons in which A(H3N2) viruses predominated, hospitalizations and deaths were more common, and the effectiveness of the vaccine was lower. Annual influenza vaccination is recommended for all persons aged ≥6 months who do not have contraindications. Multiple influenza vaccines are approved and recommended for use during the 2017-18 season, and vaccination should continue to be offered as long as influenza viruses are circulating and unexpired vaccine is available. This report summarizes U.S. influenza activity* during October 1-November 25, 2017 (surveillance weeks 40-47).†.
Conflict of interest statement
Figures

References
-
- Association of Public Health Laboratories. Influenza virologic surveillance right size roadmap. 1st ed. Silver Spring, MD: Association of Public Health Laboratories; 2013. https://www.aphl.org/AboutAPHL/publications/Documents/ID_July2013_Influe...
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

