A Randomized Trial Evaluating the Prophylactic Activity of DSM265 Against Preerythrocytic Plasmodium falciparum Infection During Controlled Human Malarial Infection by Mosquito Bites and Direct Venous Inoculation
- PMID: 29216395
- PMCID: PMC5853383
- DOI: 10.1093/infdis/jix613
A Randomized Trial Evaluating the Prophylactic Activity of DSM265 Against Preerythrocytic Plasmodium falciparum Infection During Controlled Human Malarial Infection by Mosquito Bites and Direct Venous Inoculation
Erratum in
-
Erratum.J Infect Dis. 2018 Jul 2;218(3):508. doi: 10.1093/infdis/jiy351. J Infect Dis. 2018. PMID: 29982801 Free PMC article. No abstract available.
Abstract
Background: DSM265 is a selective inhibitor of Plasmodium dihydroorotate dehydrogenase that fully protected against controlled human malarial infection (CHMI) by direct venous inoculation of Plasmodium falciparum sporozoites when administered 1 day before challenge and provided partial protection when administered 7 days before challenge.
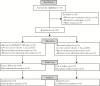
Methods: A double-blinded, randomized, placebo-controlled trial was performed to assess safety, tolerability, pharmacokinetics, and efficacy of 1 oral dose of 400 mg of DSM265 before CHMI. Three cohorts were studied, with DSM265 administered 3 or 7 days before direct venous inoculation of sporozoites or 7 days before 5 bites from infected mosquitoes.
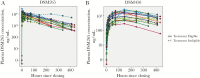
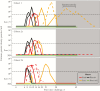
Results: DSM265-related adverse events consisted of mild-to-moderate headache and gastrointestinal symptoms. DSM265 concentrations were consistent with pharmacokinetic models (mean area under the curve extrapolated to infinity, 1707 µg*h/mL). Placebo-treated participants became positive by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and were treated 7-10 days after CHMI. Among DSM265-treated subjects, 2 of 6 in each cohort were sterilely protected. DSM265-treated recipients had longer times to development of parasitemia than placebo-treated participants (P < .004).
Conclusions: This was the first CHMI study of a novel antimalarial compound to compare direct venous inoculation of sporozoites and mosquito bites. Times to qRT-PCR positivity and treatment were comparable for both routes. DSM265 given 3 or 7 days before CHMI was safe and well tolerated but sterilely protected only one third of participants.
Keywords: CHMI; DSM265; Plasmodium; RT-PCR; preerythrocytic; prophylaxis.
© The Author(s) 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


References
-
- WHO. World Malaria Report 2016. 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

