High-Throughput H295R Steroidogenesis Assay: Utility as an Alternative and a Statistical Approach to Characterize Effects on Steroidogenesis
- PMID: 29216406
- PMCID: PMC10716795
- DOI: 10.1093/toxsci/kfx274
High-Throughput H295R Steroidogenesis Assay: Utility as an Alternative and a Statistical Approach to Characterize Effects on Steroidogenesis
Erratum in
-
Erratum to "High-Throughput H295R Steroidogenesis Assay: Utility as an Alternative and a Statistical Approach to Characterize Effects on Steroidogenesis".Toxicol Sci. 2018 Aug 1;164(2):646. doi: 10.1093/toxsci/kfy148. Toxicol Sci. 2018. PMID: 29939356 No abstract available.
Abstract
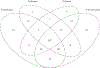
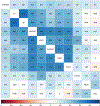
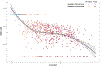
The U.S. Environmental Protection Agency Endocrine Disruptor Screening Program and the Organization for Economic Co-operation and Development (OECD) have used the human adrenocarcinoma (H295R) cell-based assay to predict chemical perturbation of androgen and estrogen production. Recently, a high-throughput H295R (HT-H295R) assay was developed as part of the ToxCast program that includes measurement of 11 hormones, including progestagens, corticosteroids, androgens, and estrogens. To date, 2012 chemicals have been screened at 1 concentration; of these, 656 chemicals have been screened in concentration-response. The objectives of this work were to: (1) develop an integrated analysis of chemical-mediated effects on steroidogenesis in the HT-H295R assay and (2) evaluate whether the HT-H295R assay predicts estrogen and androgen production specifically via comparison with the OECD-validated H295R assay. To support application of HT-H295R assay data to weight-of-evidence and prioritization tasks, a single numeric value based on Mahalanobis distances was computed for 654 chemicals to indicate the magnitude of effects on the synthesis of 11 hormones. The maximum mean Mahalanobis distance (maxmMd) values were high for strong modulators (prochloraz, mifepristone) and lower for moderate modulators (atrazine, molinate). Twenty-five of 28 reference chemicals used for OECD validation were screened in the HT-H295R assay, and produced qualitatively similar results, with accuracies of 0.90/0.75 and 0.81/0.91 for increased/decreased testosterone and estradiol production, respectively. The HT-H295R assay provides robust information regarding estrogen and androgen production, as well as additional hormones. The maxmMd from this integrated analysis may provide a data-driven approach to prioritizing lists of chemicals for putative effects on steroidogenesis.
Figures





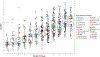
References
-
- Abdel-Khalik J, Bjorklund E, and Hansen M (2013). Development of a solid phase extraction method for the simultaneous determination of steroid hormones in H295R cell line using liquid chromatography-tandem mass spectrometry. J. Chromatog.r B Analyt. Technol. Biomed. Life Sci 935, 61–69. - PubMed
-
- Ankley GT, and Jensen KM (2014). A novel framework for interpretation of data from the fish short-term reproduction assay (FSTRA) for the detection of endocrine-disrupting chemicals. Environ. Toxicol. Chem 33, 2529–2540. - PubMed
-
- Asser L, Hescot S, Viengchareun S, Delemer B, Trabado S, and Lombes M (2014). Autocrine positive regulatory feedback of glucocorticoid secretion: Glucocorticoid receptor directly impacts H295R human adrenocortical cell function. Mol. Cell. Endocrinol 395, 1–9. - PubMed
-
- Blystone CR, Lambright CS, Howdeshell KL, Furr J, Sternberg RM, Butterworth BC, Durhan EJ, Makynen EA, Ankley GT, Wilson VS, et al. (2007). Sensitivity of fetal rat testicular steroidogenesis to maternal prochloraz exposure and the underlying mechanism of inhibition. Toxicol. Sci 97, 512–519. - PubMed
-
- Breen M, Villeneuve DL, Ankley GT, Bencic DC, Breen MS, Watanabe KH, Lloyd AL, and Conolly RB (2013). Developing predictive approaches to characterize adaptive responses of the reproductive endocrine axis to aromatase inhibition: II. Computational modeling. Toxicol. Sci 133, 234–247. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

