Access to affordable medicines and diagnostic tests for asthma and COPD in sub Saharan Africa: the Ugandan perspective
- PMID: 29216852
- PMCID: PMC5721472
- DOI: 10.1186/s12890-017-0527-y
Access to affordable medicines and diagnostic tests for asthma and COPD in sub Saharan Africa: the Ugandan perspective
Abstract
Background: Equitable access to affordable medicines and diagnostic tests is an integral component of optimal clinical care of patients with asthma and chronic obstructive pulmonary disease (COPD). In Uganda, we lack contemporary data about the availability, cost and affordability of medicines and diagnostic tests essential in asthma and COPD management.
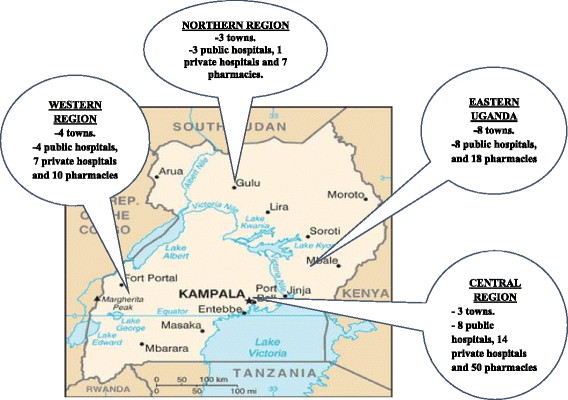
Methods: Data on the availability, cost and affordability of 17 medicines and 2 diagnostic tests essential in asthma and COPD management were collected from 22 public hospitals, 23 private and 85 private pharmacies. The percentage of the available medicines and diagnostic tests, the median retail price of the lowest priced generic brand and affordability in terms of the number of days' wages it would cost the least paid public servant were analysed.
Results: The availability of inhaled short acting beta agonists (SABA), oral leukotriene receptor antagonists (LTRA), inhaled LABA-ICS combinations and inhaled corticosteroids (ICS) in all the study sites was 75%, 60.8%, 46.9% and 45.4% respectively. None of the study sites had inhaled long acting anti muscarinic agents (LAMA) and inhaled long acting beta agonist (LABA)-LAMA combinations. Spirometry and peak flow-metry as diagnostic tests were available in 24.4% and 6.7% of the study sites respectively. Affordability ranged from 2.2 days' wages for inhaled salbutamol to 17.1 days' wages for formoterol/budesonide inhalers and 27.8 days' wages for spirometry.
Conclusion: Medicines and diagnostic tests essential in asthma and COPD care are not widely available in Uganda and remain largely unaffordable. Strategies to improve access to affordable asthma and COPD medicines and diagnostic tests should be implemented in Uganda.
Keywords: Access; Asthma; COPD; Diagnostic tests; Medicines; Sub Saharan Africa; Uganda.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval to conduct this study was granted by the ethics review board of St. Francis hospital, Nsambya Uganda as approved by the Uganda National Council of Science and Technology (UNCST).
Consent for publication
No individual person’s data in any form (details, image and videos) was used in this manuscript.
Competing interests
DK works in the medical unit of GlaxoSmithKline (GSK) pharmaceutical Kenya Limited in Uganda. GSK did not participate in the study funding, design or analysis of the data. The views expressed in this manuscript are solely the author’s (DK). The rest of the authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- WHO. Chronic respiratory diseases. 2016. http://www.who.int/respiratory/en/. Accessed 11 Jan 2017.
-
- Kirenga B, Nakiyingi L, Worodria W, Okot-Nwang M. Chronic respiratory diseases in a tertiary healthcare facility in Uganda. African journal of. Respir Med. 2013;8(2):21–23.
-
- van Gemert F, Kirenga B, Chavannes N, Kamya M, Luzige S, Musinguzi P, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health. 2015;3:e44–51. doi: 10.1016/S2214-109X(14)70337-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical