Pro-inflammatory adjuvant properties of pigment-grade titanium dioxide particles are augmented by a genotype that potentiates interleukin 1β processing
- PMID: 29216926
- PMCID: PMC5721614
- DOI: 10.1186/s12989-017-0232-2
Pro-inflammatory adjuvant properties of pigment-grade titanium dioxide particles are augmented by a genotype that potentiates interleukin 1β processing
Abstract
Background: Pigment-grade titanium dioxide (TiO2) particles are an additive to some foods (E171 on ingredients lists), toothpastes, and pharma-/nutraceuticals and are absorbed, to some extent, in the human intestinal tract. TiO2 can act as a modest adjuvant in the secretion of the pro-inflammatory cytokine interleukin 1β (IL-1β) when triggered by common intestinal bacterial fragments, such as lipopolysaccharide (LPS) and/or peptidoglycan. Given the variance in human genotypes, which includes variance in genes related to IL-1β secretion, we investigated whether TiO2 particles might, in fact, be more potent pro-inflammatory adjuvants in cells that are genetically susceptible to IL-1β-related inflammation.
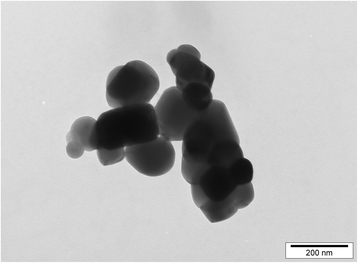
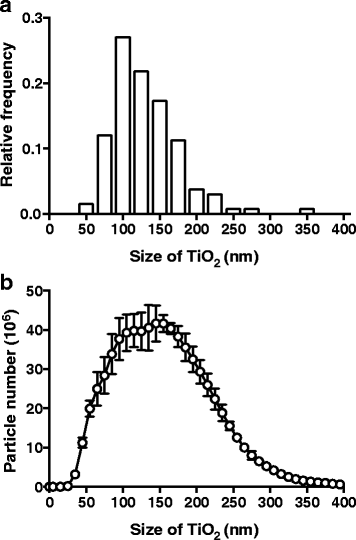
Methods: We studied bone marrow-derived macrophages from mice with a mutation in the nucleotide-binding oligomerisation domain-containing 2 gene (Nod2 m/m), which exhibit heightened secretion of IL-1β in response to the peptidoglycan fragment muramyl dipeptide (MDP). To ensure relevance to human exposure, TiO2 was food-grade anatase (119 ± 45 nm mean diameter ± standard deviation). We used a short 'pulse and chase' format: pulsing with LPS and chasing with TiO2 +/- MDP or peptidoglycan.
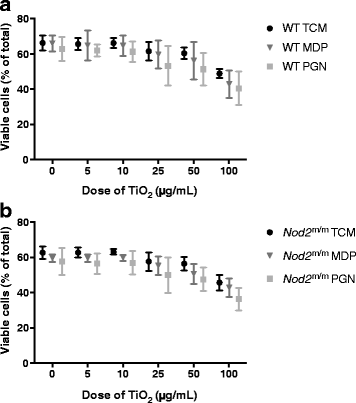
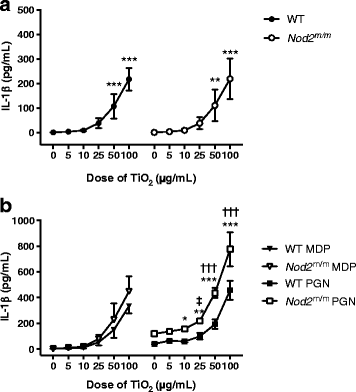
Results: IL-1β secretion was not stimulated in LPS-pulsed bone marrow-derived macrophages, or by chasing with MDP, and only very modestly so by chasing with peptidoglycan. In all cases, however, IL-1β secretion was augmented by chasing with TiO2 in a dose-dependent fashion (5-100 μg/mL). When co-administered with MDP or peptidoglycan, IL-1β secretion was further enhanced for the Nod2 m/m genotype. Tumour necrosis factor α was triggered by LPS priming, and more so for the Nod2 m/m genotype. This was enhanced by chasing with TiO2, MDP, or peptidoglycan, but there was no additive effect between the bacterial fragments and TiO2.
Conclusion: Here, the doses of TiO2 that augmented bacterial fragment-induced IL-1β secretion were relatively high. In vivo, however, selected intestinal cells appear to be loaded with TiO2, so such high concentrations may be 'exposure-relevant' for localised regions of the intestine where both TiO2 and bacterial fragment uptake occurs. Moreover, this effect is enhanced in cells from Nod2 m/m mice indicating that genotype can dictate inflammatory signalling in response to (nano)particle exposure. In vivo studies are now merited.
Keywords: E171; IL-1β; Muramyl dipeptide; NOD2; Nano; Particle; Peptidoglycan; TNF-α; TiO2.
Conflict of interest statement
Ethics approval
Collection of bone marrow from mice for this research was approved by the Grasslands Ethics Committee (Palmerston North, New Zealand), AgResearch Animal Ethics Committee, applications AE Tissue Collection 54 and 68 in compliance with the New Zealand Animal Welfare Act 1999.
Use of human blood for this research was approved by the ethics committee of the University of Cambridge (Cambridge, UK), Human Biology Research Ethics Committee, application HBREC.2015.10.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Rompelberg C, Heringa MB, van Donkersgoed G, Drijvers J, Roos A, Westenbrink S, et al. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology. 2016;10:1404–1414. doi: 10.1080/17435390.2016.1222457. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

